Abstract
Activated protein C (APC) resistance caused by the factor V Leiden mutation is associated with an increased risk of venous thrombosis. We investigated whether a reduced response to APC, not due to the factor V point mutation, is also a risk factor for venous thrombosis. For this analysis, we used the Leiden Thrombophilia Study (LETS), a case-control study for venous thrombosis including 474 patients with a first deep-vein thrombosis and 474 age- and sex-matched controls. All carriers of the factor V Leiden mutation were excluded. A dose-response relationship was observed between the sensitivity for APC and the risk of thrombosis: the lower the normalized APC sensitivity ratio, the higher the associated risk. The risk for the lowest quartile of normalized APC-SR (<0.92), which included 16.5% of the healthy controls, compared with the highest quartile (normalized APC-SR > 1.05) was greater than fourfold increased (OR = 4.4; 95% confidence interval, 2.9 to 6.6). We adjusted for VIII:C levels, which appeared to affect our APC resistance test. The adjusted (age, sex, FVIII:C) odds ratio for the lowest quartile was 2.5 (95% confidence interval, 1.5 to 4.2). So, after adjustment for factor VIII levels, a reduced response to APC remained a risk factor. Our results show that a reduced sensitivity for APC, not caused by the factor V Leiden mutation, is a risk factor for venous thrombosis.
A KEY COMPONENT in the anticoagulant pathway is protein C, the zymogen of a vitamin K-dependent serine protease. Protein C is activated on endothelial cells by thrombin bound to thrombomodulin. Activated protein C (APC) exerts its anticoagulant function by proteolytic cleavage of the procoagulant proteins factor Va and factor VIIIa. Protein S functions as a cofactor in this reaction (for a review, see Esmon and Schwarz1).
The phenomenon of resistance to APC was first reported by Dahlbäck et al in 1993.2 It is defined as a poor anticoagulant response of plasma to APC and is associated with an increased risk of thrombosis.3,4 In 1994 it was found that APC resistance is almost always associated with the presence of a mutation in one of the APC cleavage sites (Arg 506) of factor V, resulting in a mutant factor V (factor V Leiden).5 The activated factor V variant is more slowly inactivated by APC than activated wild-type factor V.6-9 APC resistance caused by the factor V Leiden mutation is a common and strong risk factor for venous thrombosis, with a threefold to sevenfold increased risk for heterozygous individuals and an 80-fold increased risk for homozygous individuals.10 11
Most cases of APC resistance can be explained by the factor V Leiden mutation. However, there are subjects who appear APC resistant as defined by arbitrary cutoff points in functional tests but who do not carry the factor V Leiden mutation. Certain acquired conditions, such as pregnancy12 and oral contraceptive use,13may account for a reduced response to APC. Patients with acute thrombotic events have also been found to have acquired APC resistance,14 which may be due to the associated acute-phase reaction. A non-factor V Leiden–related APC resistance was also reported in patients with stroke,15,16 but it is unknown if this is caused by genetic or acquired conditions. Some laboratory phenotypes, such as lupus anticoagulant17 and high factor VIII levels,18 19 are also associated with a reduced sensitivity for APC. The detection of an APC-resistant phenotype depends on the type of clotting test and on the particular reagent that is used. It is not known if this acquired APC resistance is associated with an increased risk of thrombosis.
In some patients, the APC-resistant phenotype without the factor V mutation might be related to acquired conditions; in others, there is no obvious reason. In family studies and in case-control studies it has been observed that thrombotic individuals without the factor V Leiden mutation have lower APC ratios than nonthrombotic subjects.20 21 These observations suggest that a reduced response to APC may be a risk factor per se for venous thrombosis and that the APC sensitivity ratio might constitute a useful clinical variable.
We have investigated whether a reduced sensitivity for APC, not due to factor V Leiden, is a risk factor for venous thrombosis. We also looked at the influence of certain variables (factor VIII, factor II, factor X, protein C, protein S [total and free], and fibrinogen levels and oral contraceptive use) on the APC ratio as measured by our local test. For our investigation we used a population-based case-control study on venous thrombosis (The Leiden Thrombophilia Study [LETS]).
PATIENTS AND METHODS
Subjects.
The design of our population-based case-control study (LETS) has been described previously.3 Briefly, consecutive patients with a first episode of deep-vein thrombosis were selected from the files of three anticoagulation clinics in The Netherlands. These clinics monitor the anticoagulant treatment of virtually all patients within a well-defined geographical area. All patients were younger than 70 years of age and were not diagnosed with malignant disorders. Controls were acquaintances of patients or partners of other patients, matched for age and sex with the cases. The study included 474 patients and 474 controls.
For the present investigation, all subjects with the factor V Leiden mutation (heterozygous and homozygous) were excluded (n = 106). Patients using oral anticoagulants (n = 48) or with a lupus anticoagulant (n = 4) were also excluded, because they have a prolonged activated partial thromboplastin time (APTT) in the absence of APC leading to unreliable results in the APC resistance test. After this selection, 337 patients and 455 controls were left for our analysis.
Blood collection and laboratory analysis.
Blood was collected into tubes containing 0.106 mmol/L trisodium citrate. Plasma was prepared by centrifugation for 10 minutes at 2,000g at room temperature and stored at −70°C. High molecular weight DNA was isolated from leukocytes and stored at 4°C. The presence of the factor V Leiden mutation was determined as previously described.5
The sensitivity of the plasma APTT to APC was measured as described before.3 Briefly, 50 μL undiluted plasma was incubated with 50 μL APTT reagent (Cephotest; Nycomed Pharma, Oslo, Norway) for 6 minutes at 37°C. For measuring the APTT in the absence of APC, clot formation was started with 50 μL of 33 mmol/L calcium chloride, 25 mmol/L Tris-HCl (pH 7.5), 50 mmol/L sodium chloride, and 0.05% ovalbumin (solution A). The APTT in the presence of APC was measured by adding 50 μL of solution A also containing 2.0 μg/mL human APC and 0.6% glycerol. Automated analysis was performed on an ACL-300 (Instrumentation Laboratory, Milan, Italy). To reduce between-assay variation, results are expressed as normalized APC sensitivity ratios (n-APC-SR). The APC sensitivity ratio (APC-SR) is defined as the APTT in the presence of APC divided by the APTT in the absence of APC. The normalized APC-SR is calculated by dividing the APC-SR of the patient by the APC-SR of pooled normal plasma that is measured in the same run. APC was prepared from isolated human protein C as previously described22 and stored in small amounts at −30°C in a buffer containing 50 mmol/L Tris-HCl (pH 7.5), 100 mmol/L sodium chloride, 0.1% ovalbumin, and 7.5% glycerol.
Protein C activity was measured with Coamate (Chromogenix, Mölndal, Sweden) on an ACL-200 (Instrumentation Laboratory), factor II activity was measured with a chromogenic method using S-2238 (Chromogenix) and Echis Carinatus snake venom (Sigma Chemical Co, St Louis, MO)23 on an ACL-200, and factor X antigen was measured by enzyme-linked immunosorbent assay (ELISA) with a polyclonal antibody (DAKO, Glostrup, Denmark). Total protein S was measured by polyclonal ELISA24 and free protein S was measured directly in plasma by ELISA using two monoclonal antibodies specific for free protein S (Asserachrom free protein S; Diagnostica Stago, Asnieres-sur-Seine, France).25,26 The fibrinogen concentration was determined according to method of Clauss using Dade thrombin reagent (Baxter, Miami, FL) on an Electra 1000 (MLA, Pleasantville, NY). Factor VIII coagulant activity was measured by a one-stage clotting assay with FVIII-deficient plasma and automated APTT (Organon Teknica, Durham, NC) on an Electra 1000. Factor VIII:C was expressed in units per deciliter. One unit corresponds with 0.93 international units (IU). The results of all these measurements, except those for factor X, have been reported previously.3 27-29
The technicians were at all times unaware of the status of the sample (ie, patient or control).
Statistical analysis.
Odds ratios (ORs) were calculated as estimates of the relative risk of thrombosis in the standard unmatched fashion. Ninety-five percent confidence intervals (95% CI) were constructed according to Woolf.30 We adjusted for the matching variables age and sex as well as for putative confounders by unconditional logistic regression. Factor VIII, factor II, factor X, protein C, protein S (total and free), and fibrinogen levels were entered into the logistic model as categorized variables (approximate tertiles).
RESULTS
The ratio of male to female subjects was 1:1.5 for the patients and 1:1.3 for the controls. The mean age was 47 years for both groups (range, 16 to 70 years for the patients and 16 to 73 years for the controls). The median time between the occurrence of deep-vein thrombosis and venepuncture for this study was 18 months (range, 6 to 55 months).
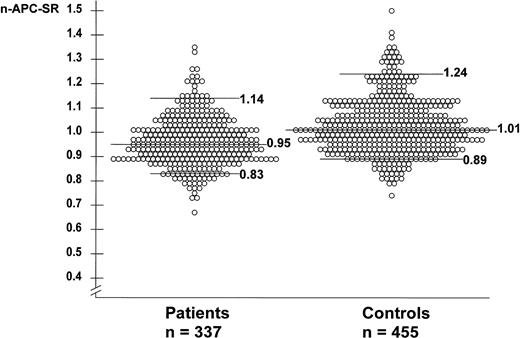
The mean n-APC-SR was 0.96 (range, 0.76 to 1.35) for the patients and 1.02 (range, 0.74 to 1.50) for the controls. The mean normalized APC-SR was higher in men than in women for patients and controls. Among the controls, the mean normalized APC-SR for men was 1.06 (range, 0.82 to 1.41) and for women 1.00 (range, 0.74 to 1.50). Among the patients, the mean normalized APC-SR for men was 0.99 (range, 0.80 to 1.26) and for women 0.94 (range, 0.67 to 1.35). The mean normalized APC-SR for women not using oral contraceptives at the time of venepuncture was slightly higher than that for the whole group of women, ie, 1.04 (range, 0.78 to 1.50) for women controls and 0.97 (range, 0.67 to 1.35) for women patients. The normalized APC-SRs of individual patients and controls are shown in Fig 1.
Normalized APC sensitivity ratios in 337 patients with deep-vein thrombosis and 455 controls. None of these subjects carries the factor V Leiden mutation. The horizontal lines represent the median n-APC-SR (0.95 for patients and 1.01 for controls), the 95th percentile (P95; 1.14 for patients and 1.24 for controls), and the 10th percentile (P10; 0.83 for patients and 0.89 for controls).
Normalized APC sensitivity ratios in 337 patients with deep-vein thrombosis and 455 controls. None of these subjects carries the factor V Leiden mutation. The horizontal lines represent the median n-APC-SR (0.95 for patients and 1.01 for controls), the 95th percentile (P95; 1.14 for patients and 1.24 for controls), and the 10th percentile (P10; 0.83 for patients and 0.89 for controls).
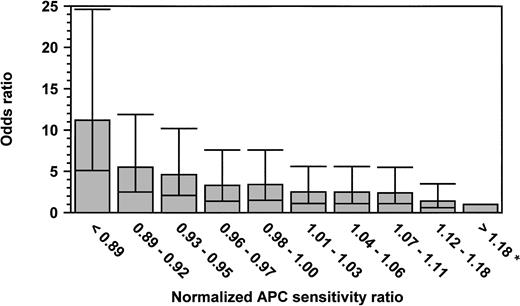
We stratified the patients and the controls into 10 groups according to the normalized APC-SRs of the controls and calculated, as a measure of the relative risk, the odds ratios (ORs) for thrombosis for the patients with lower normalized APC-SRs as compared with those with the highest normalized APC-SRs (>1.18; Fig 2). The 10th percentile of the normalized APC-SRs for controls was 0.89. Of the 337 patients, 79 (23.4%) had a normalized APC-SR below this cutoff, compared with 37 (8.1%) of the healthy controls. The 90th percentile of the normalized APC-SRs for controls was 1.18. Eleven cases (3.3%) and 48 controls (10.5%) had a normalized APC-SR above this cutoff. So, the crude OR for venous thrombosis for subjects with a normalized APC-SR below the 10th percentile was 9.3 (95% CI, 4.4 to 20) as compared with those with a normalized APC-SR above 1.18 (90th percentile). The adjusted (sex and age) OR (Fig 2) for this group was even higher, indicating an 11-fold increased risk compared with the group with the highest normalized APC-SR (OR, 11.2; 95% CI, 5.1 to 24.6). Figure 2 shows that the OR for venous thrombosis steadily increases when the normalized APC-SR decreases.
Odds ratio for venous thrombosis, according to normalized APC sensitivity ratio. ORs are adjusted for age and sex. The reference category was formed by the subjects with an n-APC-SR greater than 1.18 (OR, 1; indicated with an asterisk). The 95% CI are represented by error bars.
Odds ratio for venous thrombosis, according to normalized APC sensitivity ratio. ORs are adjusted for age and sex. The reference category was formed by the subjects with an n-APC-SR greater than 1.18 (OR, 1; indicated with an asterisk). The 95% CI are represented by error bars.
Table 1 shows the OR after stratification of the normalized APC-SRs of patients and controls into quartiles. The ORs are relative to the reference category (OR, 1), and show for both sexes together an increased risk associated with a poorer response to APC. Subjects with a normalized APC-SR below 0.92 had a 4.4-fold higher risk than those in the reference category (n-APC-SR >1.05). Because women appeared to have a lower average normalized APC-SR than men, we also performed the stratification and calculation of the ORs for men and women separately (ie, with sex-specific quartile cut-off values). For both sexes similar results were obtained with a fourfold to fivefold increased risk in the lowest quartile.
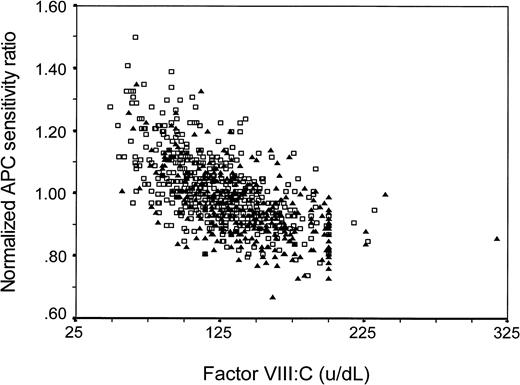
Because APC sensitivity, as measured with our test, may be influenced by other variables (eg, factor VIII levels), we assessed whether the effects of these other variables might explain the observed association, ie, whether the association was confounded. Adjustment for protein C, protein S (free or total), factor X, factor II, or fibrinogen levels or for oral contraceptive use at the time of venepuncture did not change the reported OR for the lowest quartile (Table 2). To act as a confounder, a variable has to be associated to the putative risk factor and be a risk factor itself. As we showed previously, high factor VIII:C levels increase the risk of thrombosis,27 and, as is shown in Fig 3, factor VIII:C levels and normalized APC-SR are indeed correlated (regression coefficient β = −.002). When we adjusted for factor VIII:C levels by logistic regression, the OR decreased. This confounding was only partial, because the risk associated with normalized APC-SR remained clearly elevated after adjustment for factor VIII:C (Table 3). Entering of factor VIII:C into the logistic model as a continuous variable gave similar results as entering it as a categorized variable. Of all 197 subjects with an APC response in the lowest quartile (<0.92), 45% (28 controls and 60 patients) had a high factor VIII:C level (>150 IU/dL, as defined by Koster et al27). The adjusted (sex, age, and FVIII:C) OR for the group below the 10th percentile (n-APC-SR <0.89) compared with the group above the 90th percentile (n-APC-SR >1.18) was 5.4 (95% CI, 2.3 to 12.8).
Factor VIII:C level and normalized APC sensitivity ratio in patients (▴) and controls (□). Regression coefficient β = −.002.
Factor VIII:C level and normalized APC sensitivity ratio in patients (▴) and controls (□). Regression coefficient β = −.002.
DISCUSSION
APC resistance caused by the factor V Leiden mutation is a strong risk factor for venous thrombosis.10 11 Our study shows that a reduced sensitivity for APC in the absence of the factor V Leiden mutation is also associated with an increased risk of venous thrombosis. This association appeared to have a dose-response relationship, ie, the lower the normalized APC sensitivity ratio, the higher the risk. As our results show, this reduced sensitivity for APC is a common risk factor for thrombosis: the risk adjusted for factor VIII in the lowest quartile, ie, with a sensitivity that has a prevalence of 25%, was 2.5-fold increased.
The lower response of patients compared with controls may reflect a postthrombotic effect, but the median time between thrombotic event and venepuncture for our study was 18 months, which makes this explanation unlikely.
It has been demonstrated previously that a commercial APC resistance test (Chromogenix) is sensitive for factor VIII levels.18,19 We showed that high factor VIII levels also influence our local APC resistance test. After stratification of the normalized APC-SRs into quartiles, we observed that 45% of the subjects in the lowest quartile (n-APC-SR <0.92) had high factor VIII:C levels (>150 IU/dL). High levels of factor VIII are a risk factor for venous thrombosis.27 After correction for the confounding effect of factor VIII levels, a low response to APC remained, although attenuated, a marked risk factor for venous thrombosis. Other variables (factor II, factor X, fibrinogen, protein S [free and total], and protein C concentrations) did not influence the reported odds ratios. The established increased risk was also not confounded by oral contraceptive use, another risk factor for venous thrombosis. Even though oral contraceptive users have lower normalized APC-SRs than nonusers, thrombosis patients using oral contraceptives still have lower ratios than controls using oral contraceptives.31
The APTT-based APC resistance test detects states with low or high sensitivity for the degradation of factor Va and VIIIa by APC. Factor V Leiden is known to be responsible for most cases of low sensitivity for APC. After the discovery of the factor V Leiden mutation, the APC resistance test has been widely used as a screening assay for this point mutation, whereby an arbitrarily chosen cutoff point is used to identify subjects to be APC resistant. Modified tests (eg, dilution in factor V-deficient plasma) were developed with a high sensitivity and specificity for the factor V Leiden mutation. An advantage of these tests is that they can also be used for the detection of the factor V mutation in patients using oral anticoagulants or in patients with lupus anticoagulants. A disadvantage of these tests is that they cannot detect a reduced sensitivity for APC in the absence of factor V Leiden, which, as we have shown here, is a risk factor for venous thrombosis. By measuring the APC response with the original test, valuable information is obtained concerning the protein C/protein S system across all levels, because, apart from the factor V Leiden mutation, other factors (eg, factor VIII level) determine the APC response.
It was demonstrated previously that our local APC resistance test, with Cephotest as activator, has a high sensitivity and specificity for the factor V Leiden mutation.5,32 33 Homozygotes for the factor V Leiden mutation have a normalized APC-SR of less than 0.45, heterozygotes have a normalized APC-SR of 0.45 to 0.70, and noncarriers have a normalized APC-SR of ≥0.70. So, there is no overlap in APC ratios between factor V Leiden carriers and noncarriers. Of our study population of noncarriers, only 1 patient had an aberrant ratio (n-APC-SR = 0.67) according to this distribution.
States with a low sensitivity for APC not caused by factor V Leiden may correspond to acquired conditions, as is reported for pregnancy,12 oral contraceptive use,13 and lupus anticoagulant.17 It may also be due to other inherited traits. Apart from the factor V Leiden mutation, no other common genetic causes for APC resistance have been found to date. Bernardi et al34 reported that the HR2 haplotype is associated with significantly lower APC ratios in patients and in controls. Recently, two mutations involving the Arg306 APC cleavage site of factor V were described.35,36 One mutation (Arg 306 Thr, factor V Cambridge), which was found in 1 patient and in a first degree relative, was indeed associated with APC resistance. The other mutation (Arg 306 Gly), found in Hong Kong Chinese, was reported to be not associated with APC resistance. Zöller et al21described in 1994 three families with APC resistance without the factor V Leiden mutation. No explanation for the APC resistance phenotype in these three families has been reported yet. No mutations have been found in APC cleavage sites in factor VIII.37,38 Recently, Amano et al39 demonstrated with FVIII mutants that only a double mutant FVIII, with APC cleavage sites Arg336 and Arg562 affected, resulted in an APC-resistant phenotype.
It is tempting to compare the risks observed in this study with the risk for factor V Leiden carriers. Previously, we reported an eightfold increased risk (calculated from Rosendaal et al10) for carriers of factor V Leiden versus noncarriers. This risk cannot be compared with the risk in the present study, because the reference group is different (all noncarriers of factor V Leiden vnoncarriers with high APC sensitivity ratios in this study). When we include factor V Leiden carriers in whom the normalized APC-SR was measured (81 patients and 14 controls), their risk compared with the reference group of noncarriers with a normalized APC-SR greater than 1.18 (11 patients and 48 controls; see Results) was 25.2 (95% CI, 10.6 to 60.1). This indicates that in carriers of factor V Leiden the risk is about threefold higher than in those with normal factor V and a low APC sensitivity ratio. The factor V Leiden carriers also had the lowest normalized APC-SRs, which was invariably less than 0.70, whereas only 1 noncarrier with a normalized APC-SR less than 0.89 had a ratio less than 0.70. So, another way of assessing these data is calculating the risk of normalized APC-SR less than 0.70 (95 factor V Leiden carriers, 1 noncarrier, 82 patients, and 14 controls) to those with a normalized APC-SR greater than 1.18. In this comparison, the OR becomes 25.6 (95% CI, 10.7 to 60.8), again showing that the lower the ratio, the higher the risk.
We conclude that a reduced response to APC, as measured by our local test, increases the risk of venous thrombosis. Because of the clear dose-response relationship between the normalized APC sensitivity ratio and the risk of venous thrombosis, it is difficult to assign a cutoff point that determines subjects to have this type of APC resistance. It must be emphasized that the risk for the APC resistance in factor V Leiden carriers is higher than the risk for noncarriers with a reduced normalized APC-SR.
Supported by Grant No. 95.001 from the Trombosestichting Nederland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Frits R. Rosendaal, MD, Hemostasis and Thrombosis Research Center, Department of Hematology, Leiden University Medical Center, Bldg 1, C2-R, PO Box 9600, 2300 RC Leiden, The Netherlands.