Abstract
Human immunodeficiency virus type 1 (HIV-1) envelope protein gp41 mediates viral fusion with human host cells. The peptide segment T20/DP178, located in the C-terminus of the ectodomain of gp41, interacts with the N-terminal leucine zipper-like domain on gp41 to establish the fusogenic conformation of the virus. Synthetic T20/DP178 peptide is highly efficacious in inhibiting HIV-1 infection in vitro by disrupting the transformation of fusogenic status of viral gp41; thus, it has been proposed for clinical trial. We report that synthetic T20/DP178 is a chemoattractant and activator of human peripheral blood phagocytes but not of T lymphocytes. We further demonstrate that T20/DP178 specifically activates a seven-transmembrane, G-protein–coupled phagocyte receptor for N-formylated chemotactic peptides, formyl peptide receptor (FPR). Moreover, synthetic T20/DP178 analogs lacking N-terminal amino acids acted as FPR antagonists. Our results suggest that gp41 peptides regulate phagocyte function via FPR and identify a novel mechanism by which HIV-1 may modulate innate immunity.
HUMAN IMMUNODEFICIENCY virus type 1 (HIV-1) envelope protein is synthesized in the form of a precursor, gp160, which is subsequently cleaved by proteinases to yield mature proteins gp120 and gp41.1 gp120 is attached to the extracellular domain of gp41 noncovalently and mediates viral binding to host cells through high-affinity interaction with the cellular receptor CD4, followed by interaction with chemokine receptors that act as HIV-1 fusion cofactors.2,3 The viral envelope gp41 plays a critical role in fusion between HIV-1 and host cell membranes.2,3 The ectodomain of gp41 has been structurally modeled based on the crystal structure of influenza hemagglutinin.4 The modeling predicts the gp41 ectodomain to contain two segments as extended helices. One segment in the NH2-terminus of gp41 contains a leucine zipper-like motif, whereas another segment is located in the carboxyl terminus of the gp41 ectodomain. In the absence of gp120 and the N-terminal fusion domain, the ectodomain of gp41 forms a soluble α-helical rod-like oligomer.4
A synthetic peptide (T20/DP178) corresponding to the carboxyl-terminal ectodomain sequence (amino acid residues 643-678 of the HIV-1LAI strain) has been shown to inhibit virus-mediated cell-cell fusion completely at low nanomolar concentrations.5-9It also reduced the infectious titer of cell-free virus by 10-fold at about 20 nmol/L concentration. The anti–HIV-1 activity of T20/DP178 is correlated with its ability to interfere with HIV-1 fusion/infection by competitive association with the leucine zipper region of the native viral gp41. The T20/DP178 thus blocked the conversion of the virus to its fusogenic configuration. Because of its exceptionally efficacious anti–HIV-1 activity in vitro, T20/DP178 has been proposed for clinical trial.9a However, the effect of T20/DP178 on host immune cells has not been studied.
In the course of investigating the mechanism(s) of HIV-1 infection-associated immunosuppression, we found that HIV-1 envelope proteins gp120 and gp41 both could inhibit the response of host monocytes to chemoattractants, including the bacterial-derived chemotactic peptide fMLP and a number of chemokines that have been shown to be important mediators of inflammatory and immunological responses.10,11 The inhibitory effect of HIV-1 envelope proteins on monocyte function requires the activation of protein kinase C, a mechanism resembling desensitization of the receptors for chemoattractants. We therefore proposed that the HIV-1 envelope protein-mediated receptor desensitization may be responsible for the reduced migratory response of the cells from acquired immunodeficiency syndrome (AIDS) patients to a variety of chemoattractants in vitro.12-14 To define the structural basis for the capacity of HIV-1 envelope proteins to inhibit host cells, we evaluated selected peptide segments, including T20/DP178 of gp41, for their interaction with human leukocytes. Our results demonstrate that T20/DP178 is a potent activator of a seven-transmembrane, G-protein–coupled receptor on human phagocytes, formyl peptide receptor (FPR), which mediates phagocyte migration and activation in response to chemotactic formylated peptides.
MATERIALS AND METHODS
Cells and reagents.
The T20/DP178 and its analogs were synthesized and purified by the Department of Biochemistry of Colorado State University (Fort Collins, CO), according to the published sequences5: T20/DP178: Ac-YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF-NH2; T716: Ac-LIHSLIEESQNQQEKNEQELLELDKWASLWNWF-NH2; T719: Ac-HSLIEESQNQQEKNEQELLELDKWASLWNWF- NH2; T712: Ac-LIEESQNQQEKNEQELLELDKWASLWNWF-NH2; and T914: Ac-QNQQEKNEQELLELDKWASLWNWF-NH2.
The purity was 90% or more and the amino acid composition was confirmed by mass spectrometer. The endotoxin levels in dissolved peptides were undetectable. The chemotactic peptide fMLP was purchased from Sigma (St Louis, MO). The human peripheral blood mononuclear cells were isolated from leukopacks through the courtesy of the Transfusion Medicine Department of the National Institutes of Health Clinical Center (Bethesda, MD). Monocytes were further purified (purity >90%). Human neutrophils were purified from the same leukopacks with 3% dextran sedimentation with a purity of greater than 98%. Rat basophilic leukemia cells stably transfected with an epitope tagged receptor for chemotactic formyl peptides, FPR,15 were a kind gift of Drs H. Ali, R. Richardson, and R. Snyderman (Duke University, Durham, NC). The cells were designated ETFR and were grown in Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal calf serum (FCS), and 0.8 mg/mL geneticin (G418) to maintain selection pressure.
Chemotaxis assays.
The cell migration was assessed using a 48-well microchemotaxis chamber technique. Different concentrations of stimulants were placed in the wells of the lower compartment of the chamber (Neuro Probe, Cabin John, MA), and the cell suspension was seeded in the wells of the upper compartment, which was separated from the lower compartment by polycarbonate filters (Osmonics, Livermore, CA; 5-μm pore size for monocytes and neutrophils and 10-μm pore size for ETFR cells). The filters for ETFR cell migration were precoated with 50 μg/mL collagen type I (Sigma) to favor the attachment of the cells. After incubation at 37°C (90 minutes for monocytes, 60 minutes for neutrophils, and 300 minutes for ETFR cells), the filters were removed and stained, and the cells migrating across the filters were counted by light microscopy after coding the samples. The experiments were performed at least 5 times with each cell type, and the results are presented as the number of migrating cells per high power field or as chemotaxis indeces (CI) representing the fold increase in the number of migrating cells in response to stimuli, over the spontaneous cell migration (in response to control medium). The significance of the increase in cell migration was analyzed with the Student’s t-test, and a CI of ≥2 is statistically significant compared with medium control (at least P < .05).
Calcium mobilization.
Calcium mobilization was assayed by incubating 107/mL of monocytes, neutrophils, or ETFR cells in loading buffer containing 138 mmol/L NaCl, 6 mmol/L KCl, 1 mmol/L CaCl2, 10 mmol/L HEPES (pH 7.4), 5 mmol/L glucose, and 0.1% bovine serum albumin (BSA) with 5 μmol/L Fura-2 (Sigma) at 37°C for 30 minutes. The dye-loaded cells were washed and resuspended in fresh loading buffer. The cells were then transferred into quartz cuvettes (106 cells in 2 mL) that were placed in a luminescence spectrometer LS50 B (Perkin-Elmer Ltd, Beaconsfield, UK). Stimulants at different concentrations were added in a volume of 20 μL to the cuvettes at the indicated time points. The ratio of fluorescence at 340 and 380 nm wavelength was calculated using the FL WinLab (Perkin-Elmer Ltd) program. The assays were performed at least 5 times and results from representive experiments are shown.
Phosphorylation of FPR and measurement of MAP kinase.
Phosphorylation of FPR was examined by culturing [32P]-orthophosphate (Amersham, Arlington Heights, IL) -labeled ETFR cells (3 × 106) in 100-mm tissue culture dishes as described.15 After stimulation with fMLP or T20/DP178, the cells were lysed and immunoprecipitation was performed by using an anti-HA antibody (12CA5; Boehringer Mannheim, Indianapolis, IN) and protein G sepharose (Pharmacia, Uppsala, Sweden). The immune complexes then were eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (Novex, San Diego, CA) and subjected SDS-PAGE and autoradiography.
MAP kinase activation was measured as described previously.16 Briefly, after stimulation of human monocytes with T20/DP178, the cells were solubilized and a specific anti-p38 mitogen-activated protein kinase (MAPK) antibody (New England Biolabs, Beverly, MA) was added to the soluble fraction, followed by the addition of protein A-Sepharose (Pharmacia). The immune complexes bound to sepharose were eluted with SDS-PAGE loading buffer. After electrophoresis, the immune complexes were electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Corp, Bedford, MA) that were reacted with a mouse antiphosphotyrosine antibody followed by a horseradish peroxidase-coupled antimouse antibody (1:5,000 dilution; New England Biolabs Inc). The MAPK was finally detected by enhanced chemiluminescence (ECL; Amersham).
Binding assays.
Tritiated (3H) fMLP was purchased from Dupont NEN (Boston, MA). A single concentration of 3H-fMLP (1 nCi) was added simultaneously with different concentrations of unlabeled agonists into 200 μL of cell suspension (2 × 106 phagocytes or ETFR cells) in duplicate samples. The samples were incubated under constant rotation for 20 minutes at 37°C. After incubation, the samples were filtered onto Whatman GF/C fiber discs (Whatman International Ltd, Kent, UK) on a 12-well manifold followed by extensive washing with ice-cold phosphate-buffered saline (PBS). The paper discs were air-dried at 65°C and then were submerged in liquid scintillation cocktail and counted for β emission. The binding assays were performed 3 times. The rate of inhibition was calculated by the formula: 1 − (cpm obtained in the presence of unlabeled agonist)/(cpm in the absence of unlabeled agonist) × 100%.
RESULTS
T20/DP178 is a chemoattractant and activator of monocytes and neutrophils.
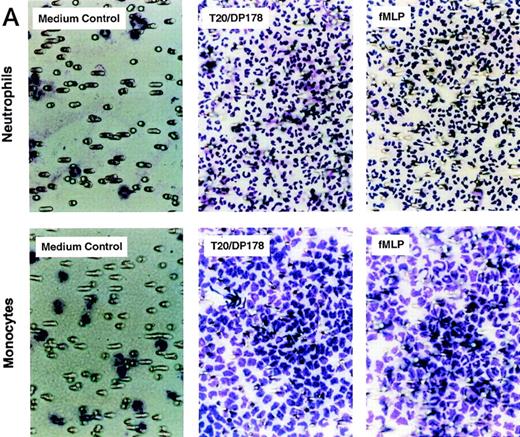
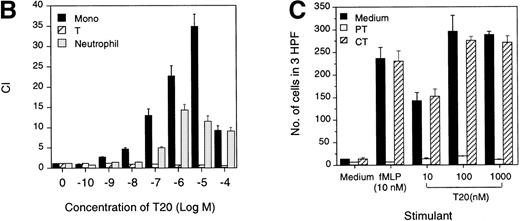
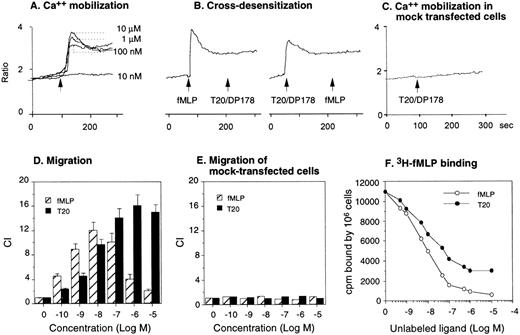
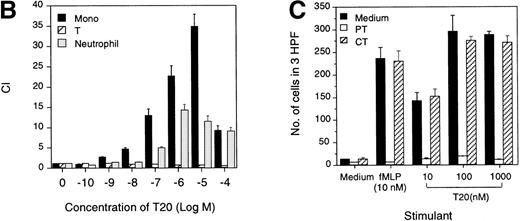
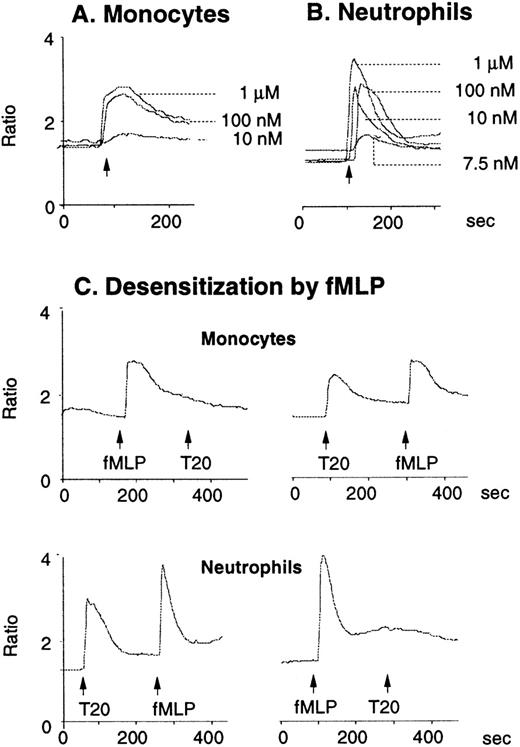
The synthetic T20/DP178 exhibited potent inhibition on fusion of both T-lymphocyte tropic and monocyte tropic HIV-1, as reported previously (Lawless et al5 and data not shown). Its analogs T716, T719, and T712 showed significant but progressively reduced anti–HIV-1 activity, whereas T914 was ineffective on HIV-1 fusion (Lawless et al5 and data not shown). The biological effects of T20/DP178 were first investigated for their ability to induce human leukocyte migration, a crucial step for cell homing and accumulation at sites of inflammation or injury. This was measured by testing the migratory response of the cells to concentration gradients of the peptide placed in the bottom wells of a micro-chemotaxis chamber. As shown in Fig 1A and B, human peripheral blood monocytes and neutrophils, but not CD3+ T lymphocytes, migrated in a dose-dependent manner in response to T20/DP178. The dose-response curve was bell-shaped, a typical pattern shown by known leukocyte chemoattractants, including fMLP and chemokines.17-20
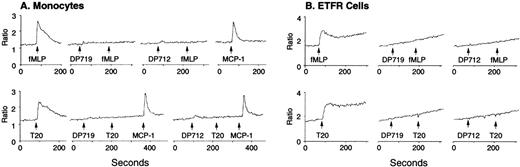
Induction of phagocyte migration by T20/DP178. Different concentrations of T20/DP178 were placed in the lower wells of the chemotaxis chamber; cell suspension was placed in the upper wells. The upper and lower wells were separated by polycarbonate filters. After incubation, the cells migrated across the filters were stained and counted. (A) Migration of neutrophils (upper panel) and monocytes (lower panel) across the filters in response to 100 nmol/L T20/DP178 or fMLP (original magnification × 200). (B) Fold increase of leukocyte migration in response to T20/DP178 over control medium. (C) Inhibition of monocyte migration in response to T20/DP178 by pretreatment of the cells with pertussis toxin (PT; 100 ng/mL for 30 minutes at 37°C). CT, cholera toxin.
Induction of phagocyte migration by T20/DP178. Different concentrations of T20/DP178 were placed in the lower wells of the chemotaxis chamber; cell suspension was placed in the upper wells. The upper and lower wells were separated by polycarbonate filters. After incubation, the cells migrated across the filters were stained and counted. (A) Migration of neutrophils (upper panel) and monocytes (lower panel) across the filters in response to 100 nmol/L T20/DP178 or fMLP (original magnification × 200). (B) Fold increase of leukocyte migration in response to T20/DP178 over control medium. (C) Inhibition of monocyte migration in response to T20/DP178 by pretreatment of the cells with pertussis toxin (PT; 100 ng/mL for 30 minutes at 37°C). CT, cholera toxin.
We next examined whether T20/DP178-induced monocyte and neutrophil migration was due to activation of specific receptors. The migration of monocytes and neutrophils to T20/DP178 was completely inhibited by pretreatment of the cells with pertussis toxin, but not cholera toxin or herbimycin A (Fig 1C), suggesting that a G-protein of the Gi-type coupled receptor was involved.17,18,20,21 This was supported by induction of a dose-dependent and pertussis toxin-sensitive calcium (Ca2+) mobilization in monocytes and neutrophils by T20/DP178 (Fig 2A and B and data not shown). The capacity of T20/DP178 to desensitize cell response to subsequent stimulation with other chemoattractants was then tested to determine whether this peptide shared a G-protein–coupled receptor(s) with other chemoattractants. T20/DP178 did not cross-desensitize the Ca2+ flux induced by a number of chemokines, including interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1), RANTES, MCP-3, or macrophage inflammatory protein-1α (data not shown), suggesting that T20/DP178 does not share a receptor with any of these chemokines. On the other hand, the bacterial chemotactic peptide fMLP had a marked desensitizing effect on T20/DP178-induced Ca2+ mobilization in both monocytes and neutrophils (Fig 2C). The desensitization of Ca2+ flux between fMLP and T20/DP178 was unidirectional when both agonists were used at same concentration (100 nmol/L), as shown in Fig 2C, because T20/DP178 had a negligible effect on subsequent fMLP stimulation. These results suggest that T20/DP178 may share only one of the two functional formyl peptide receptors (FPR and FPRL1) identified so far on human phagocytic cells.17,18 20-23
Calcium (Ca2+) mobilization in phagocytes induced by T20/DP178. Human monocytes (A) or neutrophils (B) were loaded with Fura-2 and then were stimulated with T20/DP178. The ratio of fluorescence at 340 and 380 nm wavelength was recorded and calculated using the FLWinLab program. (C) Desensitization of T20/DP178 (100 nmol/L) -induced Ca2+ flux by fMLP (100 nmol/L).
Calcium (Ca2+) mobilization in phagocytes induced by T20/DP178. Human monocytes (A) or neutrophils (B) were loaded with Fura-2 and then were stimulated with T20/DP178. The ratio of fluorescence at 340 and 380 nm wavelength was recorded and calculated using the FLWinLab program. (C) Desensitization of T20/DP178 (100 nmol/L) -induced Ca2+ flux by fMLP (100 nmol/L).
T20/DP178 is a functional ligand for formyl peptide receptor on phagocytic cells.
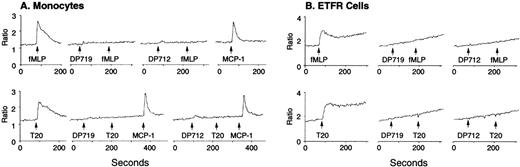
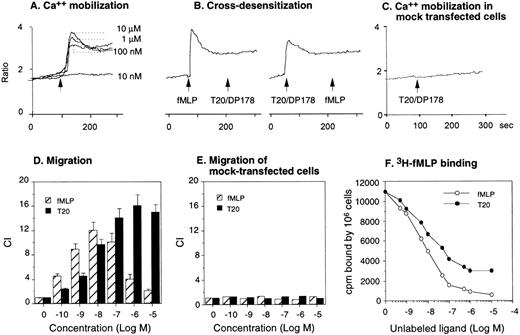
To verify whether T20/DP178 uses one of the fMLP receptors, we tested the effect of T20/DP178 on a rat basophil leukemia cell line transfected to express an epitope-tagged FPR,15,24 the first of the cloned seven transmembrane, G-protein–coupled receptors for fMLP.17,18,20 T20/DP178 induced a dose-dependent Ca2+ mobilization in ETFR cells (Fig 3A), with maximal stimulation at 1 μmol/L concentration. Sequential stimulation of the ETFR cells with fMLP and T20/DP178 or vice versa resulted in bidirectional desensitization, with fMLP being more efficacious, because a higher dose of T20/DP178 was required to completely abolish the cell response to fMLP (Fig 3B). Neither peptide stimulated Ca2+ flux in parental cells (data not shown) or mock-transfected cells (Fig 3C), indicating that the response was indeed mediated by the FPR in ETFR cells and that this receptor played a major role in the unidirectional desensitization between fMLP and T20/DP178 in phagocytes. Chemotaxis assays were then used as another sensitive biological parameter to assess the function of FPR in ETFR cells, as in our previous studies with chemokine receptors.25-27 ETFR cells could be induced by fMLP to migrate across polycarbonate filters coated with extracellular matrix protein, collagen type I, with an EC50 of approximately 500 pmol/L, whereas T20/DP178 induced an even more pronounced migration of the ETFR cells, although with lower potency (EC50 of 5 nmol/L) than fMLP (Fig 3D). In contrast, T20/DP178 or fMLP did not induce migration of mock-transfected cells (Fig 3E). To confirm that T20/DP178 is acting at FPR, we used ligand binding competition experiments with 3H-labeled fMLP. T20/DP178 effectively competed with 3H-fMLP for binding to ETFR cells, although with less potency compared with unlabeled fMLP (Fig 3F; IC50: T20/DP178, ∼5 nmol/L; fMLP, ∼1 nmol/L), in agreement with the chemotactic and Ca2+ mobilizing activity of both agonists for the ETFR cells. Further demonstration of the FPR agonist activity of T20/DP178 is shown by a rapid phosphorylation of FPR after stimulation of ETFR cells with T20/DP178 (Fig 4A). After 15 minutes of treatment of32P-labeled ETFR cells at 37°C with T20/DP178, immunoprecipitation with an antiepitope antibody (anti-HA, 12CA5) detected a specific 65-kD phosphorylated protein species in the cell lysates (Fig 4A), which was identical to the phospho-protein detected in fMLP-treated cells (Fig 4A), as previously reported by other investigators using the same cell line.15,24 Furthermore, T20/DP178 induced a time- and dose-dependent activation of MAPK in human monocytes (Fig 4B), a signaling event similarly initiated by fMLP.16 28
Activation of FPR by T20/DP178. The FPR-transfected rat basophil cell line ETFR was used to evaluate Ca2+mobilization induced by T20/DP178 (A) and cross-desensitization of cell signaling between T20/DP178 (1 μmol/L) and fMLP (100 nmol/L) (B). T20/DP178 did not induce Ca2+ in mock-transfected parental cells (C). (D) Induction of ETFR cell migration by T20/DP178 and fMLP. (E) T20/DP178 or fMLP did not induce significant migration of mock-transfected cells. (F) Displacement of 3H-fMLP binding to ETFR cells by T20/DP178.
Activation of FPR by T20/DP178. The FPR-transfected rat basophil cell line ETFR was used to evaluate Ca2+mobilization induced by T20/DP178 (A) and cross-desensitization of cell signaling between T20/DP178 (1 μmol/L) and fMLP (100 nmol/L) (B). T20/DP178 did not induce Ca2+ in mock-transfected parental cells (C). (D) Induction of ETFR cell migration by T20/DP178 and fMLP. (E) T20/DP178 or fMLP did not induce significant migration of mock-transfected cells. (F) Displacement of 3H-fMLP binding to ETFR cells by T20/DP178.
FPR phosphorylation and MAP kinase activation induced by T20/DP178. FPR phosphorylation was measured by incubating [32P]-orthophosphate–labeled ETFR cells with medium alone or T20/DP178 at 37°C for 15 minutes, and the same amount of cell lysate for each treatment was immunoprecipitated with an antiepitope (anti-HA) antibody (A). MAPK activation was examined in human monocytes by treatment of the cells with different concentrations of T20/DP178 at various time intervals and immunoprecipitation of the cell lysates with an anti-p38 MAPK antibody, followed by immunoblotting with a mouse antiphosphotyrosine antibody (B). In time-course experiments of MAPK activation, 10 μmol/L T20/DP178 was used.
FPR phosphorylation and MAP kinase activation induced by T20/DP178. FPR phosphorylation was measured by incubating [32P]-orthophosphate–labeled ETFR cells with medium alone or T20/DP178 at 37°C for 15 minutes, and the same amount of cell lysate for each treatment was immunoprecipitated with an antiepitope (anti-HA) antibody (A). MAPK activation was examined in human monocytes by treatment of the cells with different concentrations of T20/DP178 at various time intervals and immunoprecipitation of the cell lysates with an anti-p38 MAPK antibody, followed by immunoblotting with a mouse antiphosphotyrosine antibody (B). In time-course experiments of MAPK activation, 10 μmol/L T20/DP178 was used.
Analogs of T20/DP178 are antagonists of formyl peptide receptor.
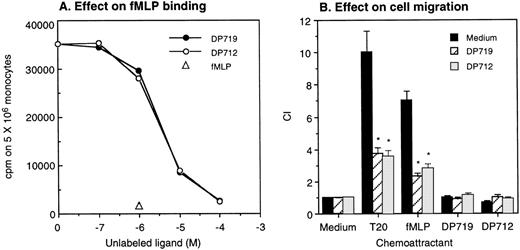
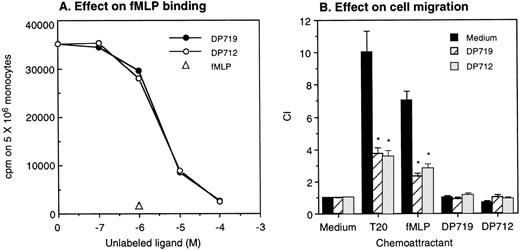
A number of FPR agonistic peptides have been reported so far.17,18,20-22 However, T20/DP178 derived from HIV-1 gp41 does not bear any substantial sequence identity with the reported FPR agonistic peptides, including the absence of an N-formyl group, suggesting that it is the conformation, rather than the primary sequence, that determines its activity. We additionally tested 4 synthetic T20/DP178 analogs that lack 3 (T716), 5 (T719), 7 (T712), and 12 (T914) amino acids, respectively, at N-terminus of the T20/DP178 and exhibit progressively reduced anti–HIV-1 efficacy in vitro.5 All of these analogs failed to induce significant Ca2+ mobilization in monocytes (Fig 5A) or ETFR cells (Fig 5B and data not shown). Instead, they abolished Ca2+ mobilization in response to subsequent T20/DP178 or fMLP stimulation in both types of phagocytic leukocytes and ETFR cells (Fig 5A and B), whereas they did not affect monocyte response to chemokines such as MCP-1 (Fig 5A). The synthetic T20/DP178 analogs also specifically inhibited3H-fMLP binding (Fig 6A) and significantly attenuated migratory response of ETFR cells induced by T20/DP178 and fMLP (Fig 6B). These results indicate that deletion of 3 to 12 amino acids from the N-terminus of T20/DP178 yielded FPR antagonists.
T20/DP178 analogs attenuate fMLP- or T20/DP178-induced Ca2+ mobilization. Monocytes (A) or ETFR cells (B) were loaded with Fura-2 and then were sequentially stimulated with T20/DP178 analogs T719 or T712 (50 μmol/L) and fMLP (100 nmol/L) or T20/DP178 (100 nmol/L). Chemokine MCP-1 (100 nmol/L) was used to indicate the specificity of T719 or T712 (A).
T20/DP178 analogs attenuate fMLP- or T20/DP178-induced Ca2+ mobilization. Monocytes (A) or ETFR cells (B) were loaded with Fura-2 and then were sequentially stimulated with T20/DP178 analogs T719 or T712 (50 μmol/L) and fMLP (100 nmol/L) or T20/DP178 (100 nmol/L). Chemokine MCP-1 (100 nmol/L) was used to indicate the specificity of T719 or T712 (A).
T20/DP178 analogs inhibit fMLP binding and abolish cell migration in response to T20/DP178 or fMLP. Different concentrations of DP719 or DP712 were added to aliquots of human monocytes containing a constant concentration of 3H-fMLP. After incubation at 37°C for 20 minutes, the cells were harvested and measured for β emission (A). Unlabeled fMLP at 1 μmol/L was used as a positive control. (B) T20/DP178 (1 μmol/L) or fMLP (100 nmol/L) was placed in the lower wells of the chemotaxis chamber; ETFR cells at 1 × 106/mL in the presence or absence of 50 μmol/L DP917 or DP912 were placed in the upper wells. After incubation, the cells that migrated across the filters were counted. DP917 or DP912 did not affect the cell migration in response to medium alone. DP917 or DP912 also did not by themselves induce ETFR cell migration. *P < .01 (Student’s t-test) compared with the migration of cells incubated with medium alone.
T20/DP178 analogs inhibit fMLP binding and abolish cell migration in response to T20/DP178 or fMLP. Different concentrations of DP719 or DP712 were added to aliquots of human monocytes containing a constant concentration of 3H-fMLP. After incubation at 37°C for 20 minutes, the cells were harvested and measured for β emission (A). Unlabeled fMLP at 1 μmol/L was used as a positive control. (B) T20/DP178 (1 μmol/L) or fMLP (100 nmol/L) was placed in the lower wells of the chemotaxis chamber; ETFR cells at 1 × 106/mL in the presence or absence of 50 μmol/L DP917 or DP912 were placed in the upper wells. After incubation, the cells that migrated across the filters were counted. DP917 or DP912 did not affect the cell migration in response to medium alone. DP917 or DP912 also did not by themselves induce ETFR cell migration. *P < .01 (Student’s t-test) compared with the migration of cells incubated with medium alone.
DISCUSSION
Our study provides novel evidence that HIV-1 envelope gp41 contains domains that are able to interact with a classical nonchemokine chemoattractant receptor on host phagocytes. This may raise a question as to what extent HIV-1 could use normal molecules on cells presumably for its advantage and to elicit a host response. Like all viruses, HIV-1 genome encodes maximal information in a minimal space. Meanwhile, unlike other retroviruses, HIV-1 has more than gag, pol, and env genes, coding for additional proteins by overlapping open reading frames and fusing exons.29 This may enable the virus to display multiple functions by processing the same protein(s), favoring the viral invasion and replication by using normal receptors on the host cells. For example, during viral fusion with host cells, HIV-1 envelope gp120, after binding to cellular CD4, interacts with a set of chemokine receptors and undergoes conformational change, enabling gp41 to present its fusion domain to the cell membrane.1-3 In addition to its pivotal role in viral fusion, gp120 and its precursor gp160 induce migration of human monocytes and CD4+ T lymphocytes, presumably through the use of CD430,31 and a chemokine receptor, CCR5.32 HIV-1 Tat protein can enhance virion replication and, in the meantime, activates a number of host cell functions, including monocyte migration and gene transcription as well as angiogenesis, via signaling through a receptor tyrosine kinase, vascular endothelial growth factor receptor-1 (VEGFR-1).33,34 Our present study demonstrates that, although envelope gp41 mediates viral fusion, its ectodomain peptide T20/DP178 is able to activate FPR on phagocytic cells. We also found that synthetic peptide corresponding to the N-terminal leucine-zipper-like domain, T21/DP107, which also posseses anti–HIV-1 fusion activity,35 preferentially activates an FPR variant FRPL1 and induces potent phagocyte migration and Ca2+ flux (Su et al35a). These observations indicate that different gp41 domains can activate multiple chemoattractant receptors that are not used by HIV-1 as fusion cofactors,2,3 but may play an important role in the recruitment and activation of phagocytic cells, thus promoting host inflammatory and innate immune responses.17,18 20-22
A major advance in the study of leukocyte motility was the discovery of synthetic N-formyl oligopeptide chemoattractants for phagocytes.36 Several natural N-formyl peptide chemoattractants, including the prototype fMLP, have since been purified from bacterial supernatants, providing evidence that they are biologically relevant ligands for FPR. Mitochondrial proteins are also N-formylated and are chemotactic for neutrophils bearing FPR,37 representing a possible source of endogenous agonist(s). Although early studies indicated that the N-formyl group was essential for optimal agonist potency,17,20,38 recent studies have shown that nonformylated peptides may also bind the FPR and activate phagocyte function.17,20,22 The synthetic pentapeptide Met-Nle-Leu-Phe-Phe-OH, either N-formylated or N-acetylated, is more potent than the parental prototype fMLP in the induction of Ca2+ flux in human neutrophils.22Amino terminal urea-substituted and carbonate-modified peptides are also potent agonists for the FPR.39,40 In addition, altering the amino acid composition of these peptides can convert an agonist to an antagonist. In our study, the non–N-formylated T20/DP178 does not bear any sequence identity to the reported FPR agonists yet showed potent FPR stimulating activity. Furthermore, the nonacetylated T20/DP178 was equally active as the acetylated form (data not shown), indicating that acetylation is not a requirement for T20/DP178 to stimulate FPR. We also observed that deletion of several amino acids from the N-terminus of T20/DP178 yielded FPR antagonists, some of which still maintained significant anti–HIV-1 fusion efficacy.5Thus, the spectrum of interaction between FPR and its agonists or antagonists appears to be broader than expected.
The binding of FPR by agonists, including fMLP, results in a cascade of G-protein–mediated signaling events leading to phagocytic cell adhesion, chemotaxis, release of oxygen intermediates, enhanced phagocytosis, and bacterial killing, as well as MAP kinase activation and gene transcription.16-18,20 Cell activation through FPR can also lead to desensitization of a subsequent cell response to other G-protein receptor ligands, including chemokines, presumably by protein kinase-mediated receptor phosphorylation.15,24 It has been reported that monocytes and neutrophils isolated from HIV-1–infected patients responded poorly to a variety of chemoattractants, including fMLP12-14 in vitro. Neutrophils from HIV-1–infected patients were reported in an activated state, yet with reduced expression and function (chemotaxis) of two receptors for chemokine IL-8, CXCR1 and CXCR2.41 Whether this reduced phagocyte migration is caused by a desensitizing effect of epitopes of HIV-1 gp41 on N-formyl peptide receptors such as FPR needs further clarification. Interestingly, in another study, we found that recombinant ectodomain of HIV-1 gp41 inhibited the response of normal human monocytes to fMLP and a variety of chemokines.11 The inhibitory effect of gp41 on monocytes was apparently due to a mechanism resembling protein kinase C-mediated receptor desensitization and surprisingly required the presence of CD4.11 Because the concentrations of gp41 we were able to achieve in that study were not more than 50 nmol/L, which did not induce Ca2+ flux and chemotaxis in monocytes, it is not clear whether at higher concentrations the full ectodomain of gp41 could directly interact with formyl peptide receptors on monocytes. Alternatively, the interaction of gp41 with cellular CD4 may also be required for its conformational change, leading to the exposure of epitopes to formyl peptide receptors. These possibilities are under rigorous investigation in this laboratory.
Although the effect of gp41 and its epitopes on immune cells in vivo is yet to be clarified, the available evidence suggests that gp41 interacts with host cells and induces potent host antibody responses. During viral fusion, the envelope gp120 is proteolytically cleaved from gp41 after binding to cellular coreceptors, leading to the exposure of gp41,1-4 which presents a fusion domain to the cell membrane. In this process, T20/DP178 and T21/DP107 epitopes play a crucial role in the transformation of gp41 to a fusogenic state.4,5 In addition, anti-gp41 antibodies can be detected in early stages of HIV-1 infection, when the viral titer has passed its peak.42 We observed that both synthetic T20/DP178 and T21/DP107 could be recognized by sera of HIV-1–infected patients in immunoblotting (data not shown), suggesting that the epitopes of gp41 are likely accessible to host immune cells, especially antigen-presenting cells.
T20/DP178 has been proven effective as an anti–HIV-1 agent in animal models (Dimitrov and Broder2 and Lambert et al42a), and its clinical trial has been proposed. Our present study calls for a more careful evaluation of the impact on the host immune system during the administration of this peptide or its analogs. The potent phagocyte activating property of T20/DP178 may promote the host innate immune response that is required for the destruction and clearance of infectious agents. However, stimulation of monocytes, in particular FPR-mediated NF-kB activation,43may also make host cells more supportive for HIV-1 replication. In this context, the ability of FPR antagonists that do not trigger cellular signaling but are able to inhibit HIV-1 envelope fusion may provide attractive candidates for anti–HIV-1 as well as anti-inflammation drug design. (After submission of this report, the results of a first and relatively short-termed clinical trial of T20/DP178 in AIDS patients were published showing a potent inhibition of HIV-1 replication by administration of T20/DP178.44 However, the effect of T20/DP178 on the host immune system remains to be determined.)
ACKNOWLEDGMENT
The authors thank T. Fu, N. Dunlop, and K. Bengali for technical assistance. The secretarial support from C. Fogle is gratefully acknowledged.
Supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-56000. S.B.S. is supported in part by a fellowship from the Office of the International Affairs, National Cancer Institute, National Institutes of Health.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The publisher or recipient acknowledges right of the US Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ji Ming Wang, LMI, DBS, NCI-FCRDC, Bldg 560, Room 31-40, Frederick, MD 21702-1201; e-mail:wangji@mail.ncifcrf.gov.


![Fig. 4. FPR phosphorylation and MAP kinase activation induced by T20/DP178. FPR phosphorylation was measured by incubating [32P]-orthophosphate–labeled ETFR cells with medium alone or T20/DP178 at 37°C for 15 minutes, and the same amount of cell lysate for each treatment was immunoprecipitated with an antiepitope (anti-HA) antibody (A). MAPK activation was examined in human monocytes by treatment of the cells with different concentrations of T20/DP178 at various time intervals and immunoprecipitation of the cell lysates with an anti-p38 MAPK antibody, followed by immunoblotting with a mouse antiphosphotyrosine antibody (B). In time-course experiments of MAPK activation, 10 μmol/L T20/DP178 was used.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3885/4/m_blod41119004aw.jpeg?Expires=1770176811&Signature=SkjA41NbDpcd9LvLwiY0sZumaXlHoBJd0lEbXWDVFB4LuVcXMmp0j0A4nsMgDlSV-t9EB96j64I9Ww1VPftfKdwL1~qEo-TTdFCE2JnbDcthnc5L1Y4mPCgQ5RjW40ev2SPPADMawiz3JEwTAFq~Mt4tedkqnJh33E4pt4VgafDSpRCjoT4LZ5-jJGCrqkdHxtD9qIAO0VUqRe23-YulsOmNtelHyYKOL5JFaD3mb-LAAUvIGZlw4b1MEPZksiMMICXbk31jUTVJsiyAtgWe8JrtRM-1gVGM2MTvT313bSyYdIWoiRvnML892RcU2yUL0Bqm36cKpq0e29jFAlxpxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. FPR phosphorylation and MAP kinase activation induced by T20/DP178. FPR phosphorylation was measured by incubating [32P]-orthophosphate–labeled ETFR cells with medium alone or T20/DP178 at 37°C for 15 minutes, and the same amount of cell lysate for each treatment was immunoprecipitated with an antiepitope (anti-HA) antibody (A). MAPK activation was examined in human monocytes by treatment of the cells with different concentrations of T20/DP178 at various time intervals and immunoprecipitation of the cell lysates with an anti-p38 MAPK antibody, followed by immunoblotting with a mouse antiphosphotyrosine antibody (B). In time-course experiments of MAPK activation, 10 μmol/L T20/DP178 was used.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3885/4/m_blod41119004bw.jpeg?Expires=1770176811&Signature=Kqtm-gE0ZL2ZM4eo2YBwxvZhPmO0kcB9EROEBoc7QO8sWM2HE8qWqLDMzF23nIM3Bf60ftr~gf5DPYW-vBSz1PGCj9IiH9pHmgfkjkImldxBZtp910F4MchjHpXaXgLp~P2Zo9KKZ2cNVoMTIhVZ3xXfwUHjt~nZeIF8syODIp9j94TRENN~BU48ZdBvfgL4vPtAzH2eN12fmaxy~qQMP-5waI~xcnAqguphkspihU43G4s7IjAJ6agfm8N3W3zZpjEISGAJzW8k6HfkOLk1kWGP8GvfPoIm0mI2aqxxxHfz5O5bsjWIpMmXqVTEkvLq6Ef5D91R4O8adsyKLJSKcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 4. FPR phosphorylation and MAP kinase activation induced by T20/DP178. FPR phosphorylation was measured by incubating [32P]-orthophosphate–labeled ETFR cells with medium alone or T20/DP178 at 37°C for 15 minutes, and the same amount of cell lysate for each treatment was immunoprecipitated with an antiepitope (anti-HA) antibody (A). MAPK activation was examined in human monocytes by treatment of the cells with different concentrations of T20/DP178 at various time intervals and immunoprecipitation of the cell lysates with an anti-p38 MAPK antibody, followed by immunoblotting with a mouse antiphosphotyrosine antibody (B). In time-course experiments of MAPK activation, 10 μmol/L T20/DP178 was used.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3885/4/m_blod41119004aw.jpeg?Expires=1770176812&Signature=l9JTpSzXM7ApohsR6ymU~IWBi-Br~UOpomDx5TpnB-XhoGhP9iZ-VdUuauc83bfamYSBiVAz~WqTgi6WzahCLyeLyFTolj3ZXP9nkl1OGs4~tQBgDtjhqRJeF2jnvZIjMJ5-IFPIOlr78~SSqXnT1Otcc5R7VgOjiIVjuPM4SPTOxsuOYPbQ~Oz5LPrYeuaXwXyWwuwdBqofLOJKdFtCuwe1DkNwIPIs5b-4jybePycgSPYUy9Ip8bwMLrWd~cTMnUr6EE-U2cIMpBHTVoOOa1T9hnpVvni8C4s9p4HIJWIXQubd5jYtUH7qBoHuqwnclRpIekILpW2GE16wo7CqhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. FPR phosphorylation and MAP kinase activation induced by T20/DP178. FPR phosphorylation was measured by incubating [32P]-orthophosphate–labeled ETFR cells with medium alone or T20/DP178 at 37°C for 15 minutes, and the same amount of cell lysate for each treatment was immunoprecipitated with an antiepitope (anti-HA) antibody (A). MAPK activation was examined in human monocytes by treatment of the cells with different concentrations of T20/DP178 at various time intervals and immunoprecipitation of the cell lysates with an anti-p38 MAPK antibody, followed by immunoblotting with a mouse antiphosphotyrosine antibody (B). In time-course experiments of MAPK activation, 10 μmol/L T20/DP178 was used.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3885/4/m_blod41119004bw.jpeg?Expires=1770176812&Signature=Q1gjAPgTreDVv9qnvsPqovOyfDxvtpenfugUQU2fk93aIhLo7QivoGK8GCwDsRHUXLbqVAQaVwttp59s-gdYVmU8N1Oqt9r99CXTy~35wX7~4qvrWSTnYTFpgfhPTmDuU-24CWuxQGa6kNoG4y8MMe0daPOEKzaJlHNWFcgIC7R-IpGhsCcnsWHLeE3JexqPGkarbs8K9rOwo9TkDuKxuJ83kPfzzZ~XD3pu4cauUmTa~uh03bo8D0i-V3p6TZ~Kv1-oedaChyIHHITuzdCcLbkZJtNQA7CeoiWCcVOR4enykAWT0vE~8lNiXGrRsfPiOenp5lyuw4NtWTQSZnicHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)