Abstract
Patients with Hodgkin’s disease, which is either refractory or recurs after frontline chemotherapy with MOPP (mechlorethamine, vincristine, procarbazine, and prednisone), ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), or both regimens, generally have a poor prognosis. High-dose chemotherapy with autologous marrow or stem cell rescue (ABMT) is now a widely used salvage strategy in these patients. In this study, our objective was to determine the response rate to ASHAP (Adriamycin = doxorubicin, Solumedrol = methylprednisolone, High-dose Ara-C = cytosine arabinoside, and Platinum = cisplatinum), in a group of patients with Hodgkin’s disease with such poor risk characteristics. The treatment was intended as a brief tumor reducing program before ABMT. Fifty-six patients with diagnosed relapsed or primary refractory Hodgkin’s disease underwent this treatment. The program consisted of the administration of two cycles of ASHAP chemotherapy (doxorubicin 10 mg/m2/d intravenous (IV) continuous infusion (CI) over 24 hours, days 1 to 4; methylprednisolone 500 mg/d IV over 15 minutes daily for 5 days; cisplatinum 25 mg/m2/d IV CI over 24 hours, days 1 to 4; cytosine arabinoside 1.5 g/m2/d IV over 2 hours on day 5). After two courses of ASHAP the patients were evaluated for response, including a gallium scan test. Patients with progressive disease were taken off the study. Those with responding or stable disease received a third course of ASHAP, followed by consolidative treatment with ABMT. There were 19 complete responses (34% CR), 20 partial responses (36% PR), and 17 treatment failures, including 8 with minor responses and 9 with disease progression. Thus, in total there were 39 responses out of 56 patients (CR + PR = 70%). Myelosuppression was the main toxicity. There were no deaths due to toxicity. At this time, 23 patients are alive. There were 31 deaths due to disease progression and 2 due to other causes. The initial response to ASHAP before subsequent ABMT consolidation treatment correlated with survival. All 17 patients in whom ASHAP failed to achieve a response have died. The presence of B symptoms at relapse, and a duration of response to the last regimen of ≤6 months, predicted a poor response to ASHAP. A short program of treatment with ASHAP is an effective tumor debulking approach in patients previously treated with both or either ABVD and MOPP, before ABMT.
ALTHOUGH HODGKIN’S DISEASE is potentially curable, 30% to 40% of patients develop relapse after primary treatment.1 After the initial relapse, response rates to subsequent therapy vary from 0% to 80%, depending on prognostic factors present at the time of relapse.2Furthermore, response rates depend on the type and duration of response attained with the initial therapy.3-5 A Cancer and Leukemia Group B (CALGB) study has convincingly established the superiority of ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine)-based regimens over MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) alone in the initial treatment of advanced Hodgkin’s disease.1 In that study, patients who had progressive disease after ABVD subsequently received MOPP. Of these patients, 61% achieved a second remission, whereas only 35% of those who suffered relapse after MOPP responded to ABVD. However, other investigators have suggested that the rates of response to salvage treatment after initial therapy with MOPP, ABVD, or hybrid regimens are similar.3
Patients with relapsed Hodgkin’s disease treated with high-dose chemotherapy and hematopoietic rescue by autologous bone marrow or peripheral stem cell have an overall failure-free survival of 30% to 40% at two years.6-9 However, the clinical characteristics of these patients have been very heterogeneous, making it difficult to define which patients benefit the most from such treatment. Potentially important features that determine results after high-dose therapy and stem cell rescue include prognostic factors at the time of relapse, the type of conditioning regimen used at the time of transplant, and possibly the cytoreductive or debulking regimen used before the high-dose conditioning regimen.
In this study, we uniformly treated patients with relapsed Hodgkin’s disease, who were potential candidates to receive high-dose chemotherapy, with a novel combination called ASHAP (Adriamycin = doxorubicin, Solumedrol = methylprednisolone, High-dose Ara-C = cytosine arabinoside, and Platinum = cisplatinum) for tumor mass reduction before high-dose chemotherapy. The selection of this regimen was based on preceding results by Velasquez et al10suggesting that this combination is an active treatment of relapsing disease in patients who had previously received both alkylating agents and doxorubicin. Our aims were to determine the response rate of relapsed or refractory Hodgkin’s disease to this regimen before intense-dose consolidative ABMT (high-dose chemotherapy with autologous marrow or stem cell rescue), and to use an abbreviated number of treatments to minimize toxicity. The results show that the regimen ASHAP is an effective cytoreductive combination in patients with Hodgkin’s disease previously treated with chemotherapy.
MATERIALS AND METHODS
Patient population.
Fifty-seven patients with relapsing Hodgkin’s disease were treated with ASHAP. One patient was retrospectively diagnosed as having T-cell rich, B-cell large cell lymphoma, and was excluded from the analysis. To be enrolled in this study, patients had to be more than 15 and less than 61 years old, have a performance status ≤2 by Zubrod’s scale, and meet the following criteria: histologically proven relapsed Hodgkin’s disease, after prior chemotherapy with either or both MOPP and ABVD, or other comparable regimens; measurable tumor masses; adequate hematopoiesis defined as granulocytes ≥1500/uL, platelet count ≥100,000/uL; adequate renal function defined as creatinine ≤1.5 mg/dL; adequate liver function defined as bilirubin ≤1.5 mg/dL, serum glutamic pyruvic transaminase (SGPT) ≤ 4× upper normal limit; adequate pulmonary function defined as forced vital capacity (FVC) ≥ 70% and diffusion capacity of carbon monoxide (DLCO) ≥ 50%, unless abnormal due to Hodgkin’s disease in the lungs; and cardiac left ventricular ejection fraction ≥50%. Patients were required to have had no chemotherapy, radiation therapy, or immunotherapy for 3 weeks before start of treatment.
Evaluation.
Before therapy, all patients underwent a complete history and physical examination, including evaluation of performance status, presence of constitutional symptoms, and concurrent nonmalignant disease and its therapy. All prior anticancer treatments were recorded, with specific notation of cumulative anthracycline dose.
Laboratory studies included a complete blood count (CBC), platelet, differential, SMA-12 (glucose, blood urea nitrogen, uric acid, calcium, phosphorus, total protein, albumin, creatinine, total bilirubin, alkaline phosphatase, serum glutamic pyruvic transaminase, and lactic dehydrogenase), prothrombin time (PT), partial thromboplastin time (PTT), urinalysis, and electrolytes. Appropriate radiological and radioisotope examinations for measurement or evaluation of disease, including gallium scans, were done within 4 weeks of starting treatment. Further tests included bilateral bone marrow aspiration and biopsy, spirometry, DLCO, electrocardiogram (EKG), cardiac scan or 2-D echocardiogram, cytomegalovirus (CMV) titer, herpes simplex serology, human immunodeficiency virus (HIV) antibody, human T-cell lymphotrophic virus type-1 (HTLV-1) antibody, and serologies for hepatitis A, B, C, and Epstein-Barr virus (EBV) antibody.
During treatment all patients had weekly monitoring of their CBC, differential and platelet counts, and before each treatment cycle SMA-12, urinalysis, and electrolytes, including magnesium, were monitored. After the first two treatment cycles, any radiographic or radioisotope studies pertinent to measurable or evaluable disease were repeated, including gallium scan testing.
Treatment plan.
Patients received two cycles of ASHAP chemotherapy, consisting of doxorubicin 10 mg/m2/d intravenous (IV) continuous infusion (CI) over 24 hours, days 1 to 4, via central venous catheter (CVC); cisplatinum 25 mg/m2/d IV CI over 24 hours, days 1 to 4, also via CVC; cytosine arabinoside 1.5 g/m2 IV over 2 hours after completion of cisplatinum (day 5); and methylprednisolone 500 mg IV over 15 minutes daily × 5 days. After two cycles of ASHAP, patients underwent evaluation for response. Those with progressive disease were removed from this study. All others, including those with minor response or stable disease, underwent autologous bone marrow harvest and storage. While awaiting confirmation of marrow viability or insurance clearance, these patients received a third cycle of ASHAP. Subsequently, they received high-dose chemotherapy consolidation and hematopoietic rescue by reinfusion of their harvested marrow, according to previously published protocol.7
Statistical methods.
Complete response (CR) was defined as complete disappearance by physical exam and radiographic studies, of all measurable or evaluable disease, with no indication of recurrence before the high-dose chemotherapy consolidation. Partial response was defined as ≥50%, but <100% improvement in all measurable or evaluable disease, with no indication of tumor regrowth before high-dose chemotherapy consolidation. Overall survival was measured from the time of registration on study. Curves were constructed using the method of Kaplan and Meier.11 Differences in survival between groups according to the different covariates were analyzed by the generalized log-rank test.12 The significance of differences in the CR rates according to various features was calculated by Chi square testing.13
RESULTS
The patients had a median age of 29 years (range 18 to 56), and the group had a balanced ratio of males and females (Table 1). The clinical features of the group were generally unfavorable. The majority of patients had extensive disease at relapse, and 38% had B symptoms. Sixty-two percent had received more than one chemotherapy regimen before ASHAP, and most had received both doxorubucin and alkylator regimens. In addition, most had prior remissions of less than 12 months’ duration.
Response to ASHAP.
Nineteen patients (34%) achieved CR and 20 (36%) achieved partial response (PR) with two cycles of ASHAP for a total response rate of 70%. Eight (14%) achieved a minor response, whereas 9 (16%) had progressive disease. The latter were removed from this study and received treatment according to other protocols including autologous bone marrow transplant (3 patients), antiferritin radioactive polyclonal antibody (2 patients), and other salvage chemotherapy regimens (4 patients).
Factors predicting poor response to ASHAP were the presence of B symptoms at relapse and a duration of response to the most recent past therapy of ≤6 months’ duration. Other factors that had no significant influence on the response rate to ASHAP included stage, the number of extranodal sites, duration of initial remission, and prior radiotherapy (Table 2).
Toxicity.
There were no deaths due to toxicity of ASHAP and no grade-III or -IV nonhematologic toxicities. None of the patients suffered cardiac or renal toxicity. By the National Cancer Institute (NCI) common toxicity criteria, all patients developed grade-III or -IV neutropenia, which was reversible, and grade-II to -III thrombocytopenia, also reversible.
Overall survival (OS) analyses.
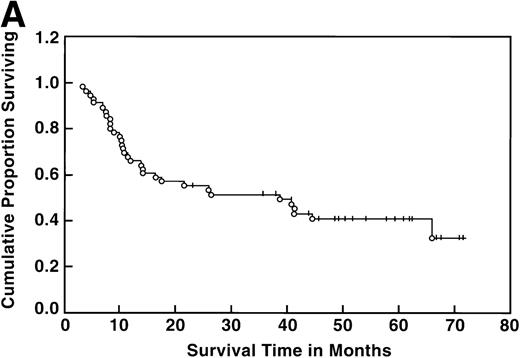
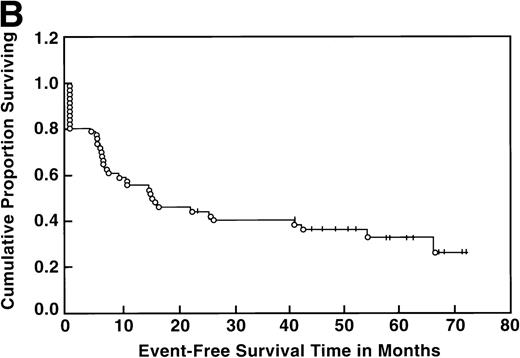
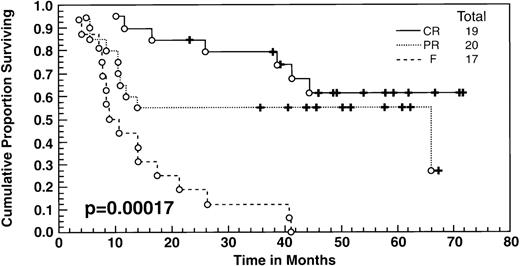
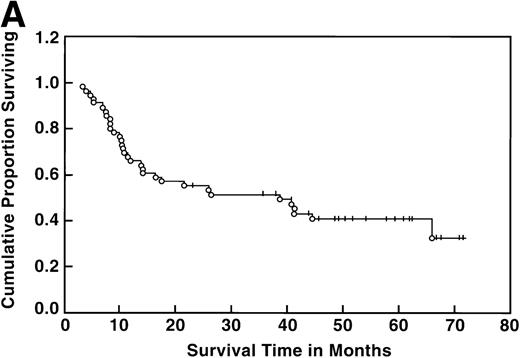
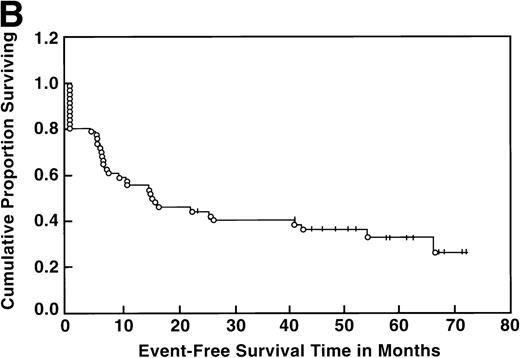
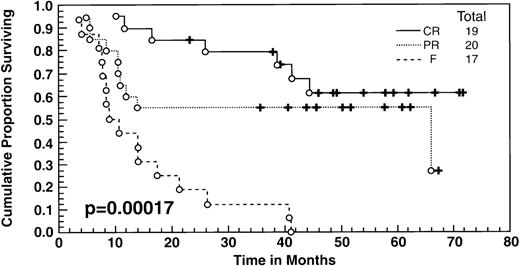
At the time of this analysis, 23 out of 56 (41%) patients were alive. Of the 33 deaths, 2 were due to causes other than Hodgkin’s disease: 1 of a heart attack, and the other a second neoplasia. The median survival and event-free survival were 37 and 16 months, respectively. At 4 years the survival was 41% (95% confidence interval [CI] 28% to 54%) and the event-free survival was 36% (95% CI, 23% to 49%) (Fig 1A, B). The response to ASHAP correlated with the long-term probability of surviving the disease, although survival in this patient population is the result of the total treatment (Fig 2). None of the 17 patients whose disease failed to respond to ASHAP (including the 8 that achieved a minor response) is alive at 4 years (P = .00017).
Overall survival (A) and event-free survival (B) in the 56 evaluable patients enrolled on study.
Overall survival (A) and event-free survival (B) in the 56 evaluable patients enrolled on study.
Overall survival according to response to ASHAP. All patients underwent ABMT after ASHAP with the exception of 2 patients who received polyclonal antiferritin antibodies, and 3 had other chemotherapy salvage regimens.
Overall survival according to response to ASHAP. All patients underwent ABMT after ASHAP with the exception of 2 patients who received polyclonal antiferritin antibodies, and 3 had other chemotherapy salvage regimens.
The mortality rate, due to causes other than disease, was 8% in the group of 47 patients who underwent the transplant after responding to ASHAP. Two patients died of acute transplant-related toxicity, 1 patient died of a heart attack, and 1 died of myelodysplastic syndrome. We performed a limited univariate analysis of recognized prognostic factors for survival in this disease other than response to salvage therapy. Specifically, we analyzed the significance of the patients’ sex and age, the stage of disease at diagnosis and relapse, duration of initial remission, and B symptoms. Only B symptoms at relapse and extranodal disease at relapse were associated with a worse outcome (P = .008 and P = .005, respectively) (Table 3).
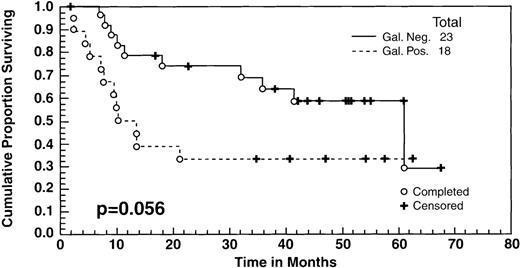
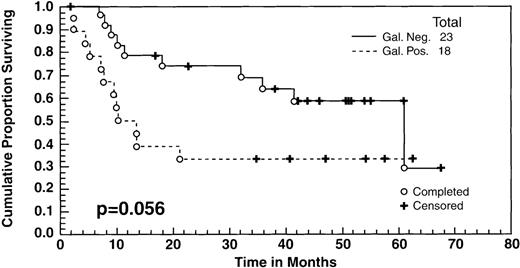
There were no significant differences in the survival between the patients who achieved a CR to ASHAP versus those who achieved a PR as measured by standard radiological studies (ie, computed tomography [CT] scans, chest radiograph [CXR]). However, as indicated in the text, there was a difference in the survival between the patients whose tumor masses became Gallium negative versus those who did not. All of the patients had positive gallium scans before entering the study, and 41 (including 19 patients in clinical CR, 20 patients in clinical PR, and 2 minor responses [MR]) had follow-up gallium scans after ASHAP. In 23 cases (57%), the gallium scan became negative after ASHAP, including all 19 patients who were deemed to be in CR and 4 additional patients whose disease response had been designated as PR by CT scans. These 23 patients with negative gallium scans had a survival rate of 60% at 5 years, compared with 35% for the 18 who still had positive gallium scans after ASHAP (P = .056) (Fig 3).
Overall survival of 41 patients who had both pre- and post-ASHAP gallium. According to gallium response, 23 became gallium negative (19 clinical CR + 4 clinical PR) whereas 18 remained gallium positive (16 clinical PR + 2 minor responses).
Overall survival of 41 patients who had both pre- and post-ASHAP gallium. According to gallium response, 23 became gallium negative (19 clinical CR + 4 clinical PR) whereas 18 remained gallium positive (16 clinical PR + 2 minor responses).
DISCUSSION
ASHAP is an active regimen in the treatment of relapsed Hodgkin’s disease. Velasquez et al10 reported a response rate of 87%, with 56% CR in 16 patients with either relapsed or refractory Hodgkin’s disease treated with ASHAP, all of whom had previously received doxorubicin-based chemotherapy. In that pilot study, patients with chemosensitive disease were administered six cycles of ASHAP. In the current report, we treated a larger number of patients and most of them (84%) had also previously received therapy with anthracyclines. Moreover, disease response in our study was assessed after only two cycles of ASHAP, with a maximum treatment of three cycles. Our goal was to debulk disease but yet minimize toxicity, anticipating further treatment with ABMT consolidation. The overall response rate was 70%, confirming the favorable activity of this drug regimen.
Other than the above study by Velasquez et al,10 there is only minimal data on the efficacy of Ara-C and Platinum in Hodgkin’s disease.14,15 The study by Rapoport et al15shows a response rate of 47% in 19 patients with relapsing Hodgkin’s disease treated with DHAP (Decadron-dexamethasone, high dose Ara-C-cytosine arabinoside, Platinum-cisplatinum) before autologous stem cell transplant. Several studies, however, have been performed with various other salvage regimens after MOPP, ABVD, or both. In the CALGB trial, 61% of patients in whom ABVD failed as initial therapy achieved a CR with MOPP, and the 3-year failure-free survival of 40% of that group is encouraging.1 However, the CALGB results are different from those reported by Viviani et al16 who reported that MOPP achieved a CR in only 25% of patients whose Hodgkin’s disease failed to respond to ABVD. Moreover, the toxicities associated with MOPP as relapse therapy, and the fact that many patients receive both MOPP and ABVD as initial therapy, make the design of alternative salvage regimens a priority goal.
The CEP regimen (CCNU [lomustine], Etoposide, and Prednimustine) produced a 30% CR rate in patients with resistant Hodgkin’s disease.17 Investigators have reported that other etoposide-based regimens, such as CEVD (lomustine, etoposide, vindesine, dexamethasone), CAV (lomustine, melphalan, etoposide) or EVA (etoposide, vinblastine, and doxorubicin), yield similar overall results.18-20 We investigated the efficacy of MIME (Methylguazone, Ifosfamide, Methotrexate, and Etoposide) in patients treated with both MOPP and ABVD or CVPP (lomustine, vinblastine, procarbazine, and prednisone)/ABDIC (doxorubicin, bleomycin, decarbazine, lomustine, prednisone).21 The CR rate in those patients was 23%. Thus, the CR rate to ASHAP in this study (34%), in patients mostly treated with both MOPP and ABVD or ABV, seems at least comparable to those reported for etoposide-based regimens. In addition, this CR rate was achieved with only two treatments, which would minimize morbidity before ABMT. Interestingly, there were no differences in the response rate to ASHAP amid the few patients who received MOPP versus those who received ABVD before ASHAP.
Four patients who had PR due to persistent masses by CT had negative gallium scans after ASHAP, whereas none of the patients who achieved clinical CR were gallium positive. The discordant readings of clinical PR and negative gallium were due to residual mass effect in areas of initially bulky disease, which can cause difficulty in distinguishing persistent active disease versus scar effect. Patients whose gallium scans became negative after ASHAP had a 60% survival rate, compared with 35% for those whose scans remained positive. On the other hand, there was no significant difference in the survival between those patients in CR versus PR to ASHAP, as defined by standard radiological studies (CXR and CT scans). Thus, our data suggests that gallium scanning is a useful adjunct to prognostic assessment of the potential benefit from high-dose chemotherapy consolidation subsequent to initial cytoreduction.
In conclusion, our study shows that ASHAP is an effective cytoreductive treatment in patients with refractory or relapsing Hodgkin’s disease before high-dose chemotherapy. It was beneficial in a group of patients who had predominantly received prior anthracycline-containing chemotherapy regimens. Treatment duration before consolidation with high-dose chemotherapy was brief, and no serious life-threatening toxicities were noted.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M. A. Rodriguez, MD, Department of Lymphoma and Myeloma, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 68, Houston, TX 77030.