In a single-center open-label prospective study, a total of 134 marrow transplant recipients with hematologic malignancies were randomly assigned to a bacterial decontamination medication using metronidazole and ciprofloxacin (n = 68) or ciprofloxacin alone (n = 66) during 5 weeks posttransplant. The development of grades II to IV acute graft-versus-host disease (GVHD) was defined as the primary study endpoint. According to the intention-to-treat, 17 patients (25%) randomized to the combined decontamination medication and 33 patients (50%) randomized to ciprofloxacin alone developed grades II to IV GVHD (P < .002). The higher frequency of grades II to IV acute GVHD in patients randomized to ciprofloxacin alone resulted from a more than twofold increased number of patients developing liver or intestinal involvement with acute GVHD compared with patients randomized to the combined decontamination medication (P < .003). The influence of the study medication on grades II to IV acute GVHD was significant only in recipients of transplants from genotypically HLA-identical sibling donors (n = 80), whereas in recipients of transplants from donors other than HLA-identical siblings (n = 54), grades II to IV acute GVHD frequencies between the study arms were not significantly different. The combined decontamination was associated with a significant reduction of culture growth of intestinal anaerobic bacteria during 5 weeks posttransplant (P < .00001). In addition, the number of cultures with growth of anaerobic bacteria (P < .005) as well as the median concentrations of anaerobic bacteria in the posttransplant period (P < .0001) were higher in patients contracting grades II to IV acute GVHD. Neither chronic GVHD nor overall survival was significantly different between the two study arms. In patients with HLA-identical sibling donors who were treated in early disease stages, the 5-year survival estimate was slightly, but not significant, higher after the combined decontamination medication (60% ± 11%) compared with ciprofloxacin alone (46% ± 9%). In conclusion, the present study provides evidence that antimicrobial chemotherapy targeted to intestinal anaerobic bacteria in marrow transplant recipients significantly reduces the severity of acute GVHD and supports the theory that the intestinal anaerobic bacterial microflora plays a role in the pathogenesis of acute GVHD after human marrow transplantation.
ACUTE GRAFT-VERSUS-HOST disease (GVHD) after allogeneic bone marrow transplantation (BMT) primarily results from an inflammatory process of recipient tissues that is induced by alloreactive donor T-cell clones recognizing differences between the marrow donor’s and recipient’s gene products encoded by the human major histocompatibility complex (MHC) or by polymorphic tissue antigens outside the MHC. Besides the outstanding significance of histoincompatibility, the development of acute GVHD is influenced and modulated by other factors, such as the type of immune prophylaxis, patient and donor age, underlying disease, and the state of alloimmunization of female donors donating marrow for male recipients.1-4
Current pathophysiologic models of acute GVHD emphasize the role of host tissue activation and damage caused by the myeloablative conditioning preceding BMT. This leads to a dysregulated release of proinflammatory cytokines and enhanced expression of tissue antigens that, in turn, may augment recognition of unshared recipient tissue antigens by alloreactive donor T cells and may amplify clonal donor T-cell expansion.5
Early studies in germ-free or completely decontaminated rodents demonstrated that the resident intestinal bacterial microflora contributes to the pathogenesis of experimental acute GVHD, because the absence or complete growth suppression of intestinal bacteria prevented the development of acute GVHD even in recipient animals of MHC-mismatched transplants.6-8 In rhesus monkeys, complete bacterial decontamination was similarly effective in preventing acute GVHD after MHC partially matched, 1 log10 T-cell depleted transplants.9 The mechanisms by which intestinal bacteria may modulate experimental acute GVHD are currently unclear. It has been hypothesized that increased cytokine release of macrophages stimulated by bacterial breakdown products, such as lipopolysaccharide (LPS) and lipoteichoic acids, or cross-reactions of donor T cells with bacterial antigens may promote the allorecognition process underlying acute GVHD.10 11
Whether the intestinal bacterial microflora is involved in the pathogenesis of acute GVHD after human marrow transplantation is currently controversial. Single small studies in leukemia patients did not find an association between intestinal bacterial growth suppression and the development of acute GVHD, whereas one randomized study in aplastic anemia patients as well as two uncontrolled trials in patients with malignant and nonmalignant hematologic diseases suggested that decontamination of intestinal bacteria reduces the incidence of grades II to IV acute GVHD.12-16
The most comprehensive analysis including 194 consecutive patients from our institution demonstrated that ineffective growth suppression of intestinal anaerobic bacteria, which represent more than 99% of the entire intestinal bacterial flora, was independently associated with a 1.7-fold higher risk of grades II to IV acute GVHD compared with sustained anaerobic bacterial decontamination during 5 weeks of the posttransplant course.16 However, only 21% of patients in this analysis achieved a decontaminated state with regard to anaerobic bacteria. We therefore asked whether the addition of oral metronidazole, which has specific bactericidal activity against anaerobic bacteria, to the broadly applied quinolone antibacterial prophylaxis in marrow transplant patients may improve anaerobic decontamination efficacy and, in turn, may reduce the incidence of grades II to IV acute GVHD. The present work describes the results obtained in an open-label prospective randomized trial that compared the influence of oral metronidazole in combination with ciprofloxacin to oral ciprofloxacin alone on the development of grades II to IV acute GVHD and on intestinal anaerobic bacterial decontamination efficacy in recipients of marrow transplants from genotypically HLA-identical sibling donors, partially HLA-matched extended family donors, or matched unrelated donors.
PATIENTS AND METHODS
Study protocol.
Adult patients (15 to 57 years of age) with hematologic malignancies for whom a genotypically HLA-identical sibling donor, a partially HLA-matched extended family donor, or a matched unrelated donor according to the German consensus criteria had been identified were eligible for this trial.17 Patients were enrolled after the scientific background, details of the study protocol, and the potential benefits and hazards of treatment in each of the two study arms had been explained and written informed consent to participate in this trial had been obtained. The study protocol had been approved by the Ethics Committee at the Medical Faculty of the University Hospital of Essen (Essen, Germany). Patient accrual was initiated in September 1993 and terminated in August 1995.
The study was performed as a single-center open-label prospective randomized trial, which compared two different strategies of intestinal bacterial decontamination. Patients randomly assigned to the combined intestinal decontamination medication were treated with oral metronidazole at 400 mg three times daily (tid) and oral ciprofloxacin at 750 mg twice daily (bid; study arm A), whereas patients assigned to ciprofloxacin alone received oral ciprofloxacin 750 mg bid (study arm B). Treatment according to study arm was initiated on day −14 (day 0 designates the day of marrow infusion) and was maintained until day 35 posttransplant, the diagnosis of acute GVHD, or death, whichever came first. Demographic and treatment characteristics of patients according to study arm are summarized in Table 1.
The development of a maximum clinical grade of severity of acute GVHD greater than grade I was defined as the primary analytical end- point of the study. The final evaluation of this endpoint was performed at 100 days posttransplant or at the time of death, if this occurred earlier. The secondary analytical study aim was a quantitative comparison of intestinal anaerobic bacterial growth suppression between the two study arms and the evaluation of the association between bacterial growth suppression and the development of grades II to IV acute GVHD.
Biometric study plan.
On the basis of our previous published analysis,16 the hypothetical difference between the two study arms with regard to the primary study endpoint was estimated to be in the range of 20%. To demonstrate a difference of this magnitude between the two study arms with a one-sided α-error of .05 and a power of 80%, a total number of 140 study patients was prospectively calculated. To allow interim analyses after 50 and 100 patients had been enrolled in the study, a group-sequential study plan was used. For rejection of the 0-hypothesis in these two interim analyses, adjusted α-errors of .0015 for the first 50 patients and of .018 for the first 100 patients were prospectively determined. These interim analyses were performed after the 50th and the 100th study patient had been observed for more than 100 days posttransplant or until death.
To ensure an equal distribution of potential influencial factors for acute GVHD between the study arms, a stratified randomization design with the following three stratification criteria was chosen: partially HLA-matched extended family donors or matched unrelated donors versus genotypically HLA-identical sibling donors, chronic myelogenous leukemia (CML) as underlying disease versus other hematologic malignancies, and female donor to male recipient transplants versus other donor and recipient gender combinations.
Statistical analysis.
Time-to-event estimates (±standard errors) were calculated by the product-limit method with right-censoring of subjects at the last time point at which they were at risk for a given event.18 For the primary study endpoint, those patients who developed graft failure or died without grades II to IV acute GVHD were censored at the respective time points of these events. Accordingly, patients surviving without grades II to IV acute GVHD were censored at the time of last follow-up.
All evaluations with regard to the primary study endpoint were based on the intent-to-treat with no exclusions due to protocol violations or drop-outs. The log-rank test was used to test the homogeneity of time-to-event distribution functions across strata.19 To evaluate the influence of different explanatory variables on the times to achieve the respective analytical endpoints, stepwise proportional hazards general linear model analyses were performed.20,21Differences between frequencies were compared using the two-tailed Fisher’s exact test (2 × 2 frequency tables) or by the Mantel-Haenszel χ2 test (2 × n frequency tables). Comparisons of continuous variables were analyzed using the Wilcoxon rank-sum test. Exponential measures were compared using the Savage test. For the comparison of timely repeated continuous measures between strata, repeated measures analysis of variance was applied.22 With the exception of the proportional hazards general linear model analyses, all indicated P values are given without adjustment for multiple testing.
Microbiologic analyses.
Fecal samples of study patients were monitored twice weekly for the growth of aerobic and anaerobic bacteria as well as fungi using modified microbiologic culture techniques as previously published.16 23 Quantitative bacterial cultures were performed using conventional plate counting technique. Standardized dilutions of stool samples were cultured on several aerobic and anaerobic culture agars (CLED agar, Clostridium difficile selective agar with D-cycloserine and cefoxitin, Mannitol Salt agar, Rose-Bangal Chloramphenicol agar, Yeast-extraxt Cystein blood agar with gentamicin and nalidixic acid, and Drigalski agar). Bacteria were categorized according to culture growth conditions as either aerobic or anaerobic. Single colonies were identified by conventional biochemical tests. Quantification of bacterial culture growth was expressed as the log10 of colony-forming units (CFU) with a detection threshold below 103 CFU per gram of sample. For the purpose of analyses on bacterial culture growth suppression, cultures with no CFU growth were calculated as 100 CFU. In patients who developed grades II to IV acute GVHD in the posttransplant course, only samples taken before the diagnosis of this condition were considered in analyses on the association of intestinal bacterial growth suppression and acute GVHD. In case of diarrhea, fecal samples were additionaly analyzed with regard to enterotoxines, enteropathic viruses, and parasites.
Supportive care.
All patients were protectively isolated in reverse isolation rooms equipped with high efficiency particular air filtration systems. These conditions were initiated on day −7 and usually maintained until day +35 posttransplant. Aseptic techniques were used during patient contacts throughout this time period. In addition to the study medication, all patients received oral or parenteral fluconazole at 200 mg bid for the prevention of candida infections. Blood component substitution, oral and parenteral nutrition, and treatment of suspected or documented bacterial or mycotic infections followed the published guidelines.16 Aciclovir treatment of suspected or documented herpes simplex virus infections was performed in 54 patients (79%) of study arm A and in 50 patients (76%) of study arm B over a median time period of 11 days (range, 3 to 20 days) and of 12 days (range, 5 to 35 days), respectively. Starting at 2 to 3 weeks posttransplant, patient peripheral blood cells were weekly monitored for replicative cytomegalovirus infections by a qualitative pp65-antigenemia assay. Based on a positive pp65-antigenemia assay, preemptive ganciclovir treatment was instituted in 27 patients (40%) of study arm A and in 27 patients (41%) of study arm B for a median treatment duration of 13 days (range, 6 to 21 days) and of 13 days (range, 7 to 21 days), respectively.
Acute adverse effects.
Acute adverse effects and organ toxicities were daily recorded and graded according to the WHO toxicity scale.24
GVHD.
Prophylaxis of acute GVHD consisted of short-course methotrexate (sMTX; 15 mg/m2 body surface area day +1, 10 mg/m2days +3, +6, and +11) and cyclosporine A (CyA; 3 mg/kg of body weight per day, continuous intravenous [IV] infusions between day −1 and day +35, oral CyA bid after day 35) in patients with partially HLA-matched family donors or matched unrelated donors, in patients with CML as underlying disease, and in male recipients of female donor transplants.1 Patients fulfilling none of these criteria exclusively received continuous CyA infusions. All but 2 patients assigned to sMTX+CyA prophylaxis received the full prescribed methotrexate dosages. Steady-state full blood levels of CyA and its major metabolites were routinely monitored twice weekly using a polyclonal antibody-based detection system (Tdx Cyclosporine and metabolites serum assay; Abbott Laboratories, Abbott Park, IL). Adjustments of CyA doses were allowed to keep blood levels within the therapeutic range of 400 to 800 ng/mL. Overall median CyA and metabolite blood levels during the first 5 weeks posttransplant were 564 ng/mL (range, 17 to 2,844 ng/mL) in 579 samples of study arm A and 530 ng/mL (range, 28 to 2,079 ng/mL) in 576 samples of study arm B (not significant [NS]).
The assessment and grading of acute and chronic GVHD was primarily based on clinical findings and followed the commonly accepted diagnostic criteria.25 26 In case of uncertain organ involvement with acute GVHD of stages 2 to 4, histologic evaluations of target organs were additionally performed. Thereby, the clinical diagnosis of acute GVHD was histologically confirmed in 28 of 89 patients (31%) with cutaneous disease (study arm A: 13 of 42 patients [31%]; study arm B: 15 of 47 patients [32%]), in 18 of 26 patients (69%) with liver disease (study arm A: 7 of 10 patients [70%]; study arm B: 11 of 16 patients [69%]), and in 17 of 26 patients (65%) with gastrointestinal disease (study arm A: 4 of 8 patients [50%]; study arm B: 13 of 18 patients [72%]).
Initial treatment of acute GVHD consisted of 2 mg/kg of body weight IV prednisolone with dose tapering according to the clinical response of acute GVHD symptoms. In patients not responding to escalated (5 to 10 mg/kg) prednisolone doses, antithymocyte globuline was used as second-line treatment.
RESULTS
Acute adverse effects.
In 63 patients (93%) of study arm A and in 59 patients (89%) of study arm B, nausea and vomiting (NAV) of grades 2 to 4 was documented (NS). In the majority of affected patients, NAV developed during the time period of myelablative conditioning. In association with the ingestion of the study medication, 21 episodes of NAV were noted in 8 patients (12%) of study arm A compared with 8 episodes in 3 patients (5%) of study arm B (NS). Oral mucositis of grades 2 to 4 developed in 56 patients (82%) of study arm A and in 61 patients (92%) of study arm B (NS). A more than 2.5-fold increase of baseline serum creatinine levels was observed in 9 patients (13%) of study arm A as opposed to 7 patients (11%) of study arm B (NS). Hepatic veno-occlusive disease (2 patients [3%] of study arm A, 3 patients [6%] of study arm B), grades 2 to 4 acute cardiac toxicity (6 patients [9%] of study arm A, 4 patients [6%] of study arm B), noninfectious interstitial pneumonia syndrome (3 patients [4%] of study arm A, 5 patients [8%] of study arm B), and multiorgan toxicity (4 patients [6%] of study arm A, 3 patients [5%] of study arm B) occurred with comparable frequencies in the two study arms. Duration of neutropenia less than 500/μL was 18 days (range, 6 to 31 days) in study arm A and 18 days (range, 10 to 30 days) in study arm B (NS). Furthermore, no differences were detectable between both study arms in the number of red blood cell and platelet transfusions, in the number of days with fever greater than 38°C and of days with IV antibiotics or antimycotics, as well as in the frequencies of documented invasive bacterial and mycotic infections (data not shown).
Primary study endpoint.
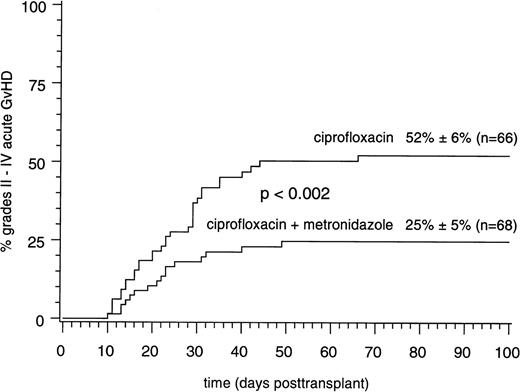
Table 2 summarizes information on the number of subjects treated according to the study protocol as well as on protocol violations and on drop-out patients in the two study arms. At the time of the first interim analysis, which included the first 50 patients, the cumulative estimates of grades II to IV acute GVHD were 18% ± 8% for study arm A and 52% ± 11% for study arm B (P < .011). Because the prospectively adjusted α-error for a premature completion of the study had not been reached by this analysis, the next 50 subjects were enrolled. The consecutive interim analysis showed cumulative estimates of grades II to IV acute GVHD of 22% ± 6% for study arm A and 54% ± 7% for study arm B (P < .0014). Because of the decrease of the α-error below .018, this analysis led to completion of the study protocol. At the time of the second analysis, an additional 34 subjects had entered the study protocol, thus resulting in a total of 134 patients included in the final evaluation (Fig 1).
Cumulative probabilities of grades II to IV acute GVHD in 134 patients randomly assigned to intestinal bacterial decontamination using ciprofloxacin (750 mg PO bid) and metronidazole (500 mg PO tid) or ciprofloxacin (750 mg PO bid) alone.
Cumulative probabilities of grades II to IV acute GVHD in 134 patients randomly assigned to intestinal bacterial decontamination using ciprofloxacin (750 mg PO bid) and metronidazole (500 mg PO tid) or ciprofloxacin (750 mg PO bid) alone.
The influence of the study arm on grades II to IV acute GVHD was significant only in recipients of transplants from HLA-identical sibling donors, with estimates of 18% ± 6% for study arm A compared with 54% ± 8% for study arm B (P < .0005). In contrast, recipients of transplants from partially HLA-matched family donors or matched unrelated donors had comparable estimates of grades II to IV acute GVHD, ranging between 36% ± 9% in study arm A and 46% ± 10% in study arm B (NS).
Organ involvement with acute GVHD.
The stages of organ involvement with acute GVHD in the two study arms are included in Table 3. The most striking differences were documented in the stages of liver and intestinal involvement, but the differences between the stages of acute cutaneous GVHD also reached significance. Consequently, the number of patients with involvement of two or three organs was more than twofold higher in study arm B (P < .02). As for the primary study endpoint, differences between the study arms resulted primarily from the significantly lower frequencies of organ involvement with acute GVHD in patients with HLA-identical sibling donors (Table 4).
Intestinal bacterial decontamination and acute GVHD.
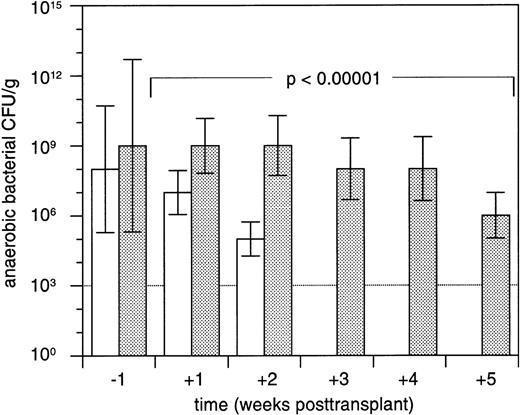
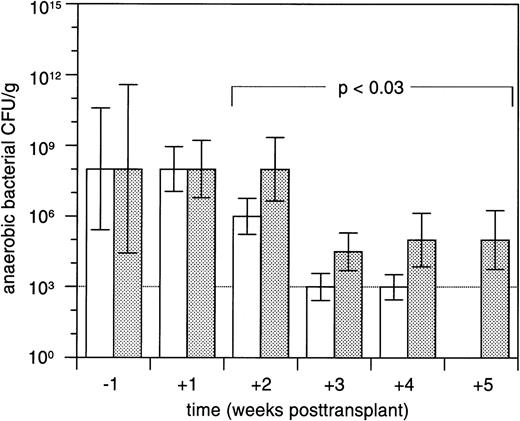
A total of 866 fecal samples were evaluable for bacterial culture growth during the posttransplant course. The proportion of fecal samples with no detectable anaerobic bacteria was more than twofold higher in study arm A (236 of 446 [53%]) compared with study arm B (96 of 420 [23%]; P < .00001). Concentrations of anaerobic CFU in the two study arms during the posttransplant course are depicted in Fig 2. In patients who contracted grades II to IV acute GVHD, the proportion of samples successfully decontaminated of anaerobic bacteria was significantly lower (80 of 257 [31%]) compared with patients with grades 0 to I acute GVHD (252 of 609 [41%]; P < .005). Whereas the concentrations of anaerobic CFU in patients who developed grades II to IV acute GVHD reached a plateau in the range of 104 to 105posttransplant, these concentrations declined in patients with grades 0 to I acute GVHD (Fig 3). Median overall concentrations of anaerobic CFU in the posttransplant course were 100-fold higher in patients contracting grades II to IV acute GVHD compared with patients with grades 0 to I acute GVHD (P < .0001).
Median concentrations (±standard errors of the median) of anaerobic bacterial CFU per gram of fecal sample in patients treated with 500 mg metronidazole PO tid and 750 mg ciprofloxacin PO bid (□; n = 68) or with 750 mg ciprofloxacin PO bid alone (▩; n = 66) in the week before and during 5 weeks after allogeneic marrow transplantation. The CFU detection threshold is 103 per gram of sample. Median concentrations of anaerobic bacterial CFU in patients treated with metronidazole and ciprofloxacin were below the detection threshold between 3 and 5 weeks posttransplant. Differences between both patient groups were compared using repeated measures analysis of variance.22
Median concentrations (±standard errors of the median) of anaerobic bacterial CFU per gram of fecal sample in patients treated with 500 mg metronidazole PO tid and 750 mg ciprofloxacin PO bid (□; n = 68) or with 750 mg ciprofloxacin PO bid alone (▩; n = 66) in the week before and during 5 weeks after allogeneic marrow transplantation. The CFU detection threshold is 103 per gram of sample. Median concentrations of anaerobic bacterial CFU in patients treated with metronidazole and ciprofloxacin were below the detection threshold between 3 and 5 weeks posttransplant. Differences between both patient groups were compared using repeated measures analysis of variance.22
Median concentrations (±standard errors of the median) of anaerobic bacterial CFU per gram of fecal sample in patients with grades 0 to I (□; n = 84) or grades II to IV (▩; n = 50) acute GVHD in the week before and during 5 weeks after allogeneic marrow transplantation. Median concentrations of anaerobic bacterial CFU in patients with grades 0 to I acute GVHD were below the detection threshold (103 per gram of sample) in week 5 posttransplant. Differences between both patient groups were compared using repeated measures analysis of variance.22
Median concentrations (±standard errors of the median) of anaerobic bacterial CFU per gram of fecal sample in patients with grades 0 to I (□; n = 84) or grades II to IV (▩; n = 50) acute GVHD in the week before and during 5 weeks after allogeneic marrow transplantation. Median concentrations of anaerobic bacterial CFU in patients with grades 0 to I acute GVHD were below the detection threshold (103 per gram of sample) in week 5 posttransplant. Differences between both patient groups were compared using repeated measures analysis of variance.22
Chronic GVHD.
Fifty-three patients in each study arm survived longer than 100 days posttransplant and were thus evaluable for chronic GVHD. The cumulative estimate of chronic GVHD was 64% ± 8% in study arm A and 70% ± 7% in study arm B (NS). Two independent predictors of chronic GVHD were identified by multivariate analysis. The sMTX+CyA regimen reduced the relative risk of chronic GVHD to 0.38 (95% confidence limits, 0.19 to 0.76) compared with CyA alone (P < .006), whereas preceding grades II to IV acute GVHD increased the relative risk to 1.80 (95% confidence limits, 1.06 to 3.06) compared with grades 0 to I acute GVHD (P < .03).
Transplant outcome.
With a median follow-up period of 51 months (range, 40 to 63 months) in both study arms, 33 of 68 (49%) patients in study arm A and 28 of 66 (42%) patients in study arm B are currently alive (NS). The 5-year survival estimates of different patient subsets are outlined in Table 5. Multivariate analysis identified two independent predictors of survival in this study. In early disease stages (ie, first complete remissions or first chronic phase), the relative survival probability was 2.02-fold (95% confidence limits, 1.27 to 3.20) higher compared with more advanced stages (P < .003). Furthermore, grades II to IV acute GVHD decreased the relative survival probability by a factor of 2.75 (95% confidence limits, 1.72 to 4.39) compared with grades 0 to I acute GVHD (P < .0001). Causes of death in the two study arms are summarized in Table 6.
DISCUSSION
Based on our previous retrospective observation that sustained growth suppression of intestinal anaerobic bacteria after allogeneic sibling donor marrow transplantation independently reduces the risk of grades II to IV acute GVHD, an open-label prospective randomized trial was conducted that compared the influence of intestinal bacterial decontamination using metronidazole combined with ciprofloxacin to ciprofloxacin alone on the development of grades II to IV acute GVHD as the primary study endpoint.16 Because of the specific bactericidal activity of metronidazole against anaerobic bacteria, it appeared justified to anticipate that the decontamination efficacy against intestinal anaerobic bacteria is much higher in patients receiving the combined medication. In consequence of our previous observation, this should result in different frequencies of grades II to IV acute GVHD in the two study arms.
In accordance with the first hypothesis, a pronounced reduction of intestinal anaerobic bacterial growth posttransplant could be demonstrated for the combined decontamination medication in comparison to ciprofloxacin alone, with differences in median concentrations of anaerobic bacteria in the range of 104 to 108. Most importantly, both study arms differed significantly with regard to the primary study endpoint, and a significant association between anaerobic bacterial growth suppression and the development of grades II to IV acute GVHD could be ascertained. It is of note that the reduction of grades II to IV acute GVHD resulted primarily from a more than twofold lower number of metronidazole-treated patients who developed liver or intestinal involvement with acute GVHD.
The biological basis by which the intestinal anaerobic bacterial microflora may modulate the development of acute GVHD after human marrow transplantation is currently unknown. Dysregulated and increased secretion of proinflammatory cytokines during the induction phase of acute GVHD is regarded as a major promoting factor of the allospecific donor T-cell activation process.5 A recent murine study supports previous clinical observations that the severity of acute GVHD increases with an intensified radiation dose of total body irradiation (TBI).27 This was associated with a systemic increase in tumor necrosis factor-α (TNF-α) levels, induced both by greater sensitivity of macrophages to LPS and by an increased translocation of LPS into the circulation of allogeneic recipient animals conditioned with the higher TBI dose. Thus, irradiation synergized with LPS stimulation of macrophages and potentiated TNF-α production in this murine model. It is therefore tempting to speculate that a substantial reduction of LPS and other soluble bacterial breakdown products by decontamination of the preponderant resident anaerobic bacterial microflora in the intestinal lumen substantially decreases entry of bacterial products into the portal and systemic circulation, which are able to induce an alloantigen-independent inflammatory process. This, in turn, would diminish or even abolish TNF-α production by monocytes and macrophages, because it is known that, in nonmalignant cells, radiation only induces TNF-α mRNA without protein production.28,29 Because TNF-α is thought to be a particularly important mediator of liver and intestinal cell damage, this hypothesis is consistent with the finding of the present study that the lower frequency of grades II to IV acute GVHD in patients treated with the combined decontamination medication is primarily a consequence of a reduced liver and intestinal involvement with acute GVHD.30-33
A further potential mechanism by which decontamination of intestinal anaerobic bacteria may modulate liver and intestinal acute GVHD is the downregulation of MHC and adhesion molecule expression on professional antigen-presenting cells (APCs) in the liver and the intestinal wall due to a reduced activation by bacterial products penetrating the intestinal mucosa.34,35 In consequence, the cellular interactions between APCs and donor T cells leading to allospecific clonal T-cell activation and expansion would not take effect. Similarly, a downregulated expression of these antigens on liver and intestinal epithelial cells would render these cells less susceptible to the cytolytic and apoptotic actions mediated by the allospecific effector cells of acute GVHD. Direct evidence for the clinical significance of these mechanisms is difficult to attain, because liver and intestinal tissue cannot be serially investigated during the early posttransplant course, and cell injury induced by TNF-α and other proinflammatory cytokines may largely depend on paracrine cytokine effects in these tissues, which are not reflected by systemic cytokine levels.36 It would therefore be interesting to study whether systemic application of neutralizing soluble receptors for bacterial LPS may be similarly effective in preventing acute GVHD after experimental and human marrow transplantation such as decontamination of the intestinal anaerobic bacterial microflora.
The influence of the combined decontamination medication on acute GVHD demonstrated for the entire study population resulted predominantly from a significant reduction of grades II to IV acute GVHD in patients with genotypically HLA-identical sibling donors, whereas in patients with donors other than HLA-identical siblings this influence was much less pronounced despite identical anaerobic bacterial decontamination efficacies (data not shown). The latter patient subset is presumed to have a higher degree of minor and major histocompatibility antigen disparity with their donors compared with recipients of HLA-identical sibling donor transplants. It can therefore be postulated that the proposed mechanisms by which anaerobic bacterial decontamination reduces acute GVHD are less effective, with an increasing degree of histoincompatibility. Possible pathophysiologic explanations for the observed differences include, among others, activation-independent expression and presentation of MHC and minor antigens by APCs in target tissues as well as enhanced allospecific donor T-cell activation due to multiple histocompatibility antigen disparities. Regardless of the quality of anaerobic bacterial decontamination, this would promote acute GVHD more strongly in recipients of transplants from partially HLA-matched family donors or matched unrelated donors compared with HLA-identical sibling donor transplant recipients.
Chronic GVHD developed with similar frequencies in the two study arms. This is not surprising, because a previous randomized trial, which compared sMTX+CyA to CyA alone for prophylaxis of acute GVHD after HLA-identical sibling marrow transplants, has demonstrated that an improvement of acute GVHD prophylaxis does not inevitably result in a reduction of chronic GVHD.1,2 The development of chronic GVHD in the present study was significantly associated with grades II to IV acute GVHD, which is in accordance with most analyses on risk factors for chronic GVHD.37 Contrary to the results of the mentioned randomized trial, the probability of chronic GVHD was significantly lower in patients treated with the combined prophylactic regimen compared with patients exclusively receiving CyA. However, in the present study, patients with donors other than HLA-identical siblings were also included and the prophylactic regimen was chosen according to risk features of acute GVHD. This heterogeneity in patient populations and treatment strategies therefore precludes a meaningful comparison of the influence of the immunoprophylactic regimen on chronic GVHD between the two studies. The documented effect of the combined decontamination medication on grades II to IV acute GVHD together with the independent influence of the sMTX+CyA regimen on chronic GVHD suggest that a combination of both strategies will synergize in reducing chronic GVHD.
Despite the significant reduction of grades II to IV acute GVHD in patients treated with the combined decontamination medication, overall survival was nearly identical in the two study arms. This analytical endpoint was adversely influenced by an advanced disease stage at the time of transplant and by the development of grades II to IV acute GVHD, both of which are generally accepted as the strongest predictors of transplant outcome. Because the effect of anaerobic bacterial decontamination on acute GVHD was largely restricted to patients with HLA-identical sibling donors, it was interesting to analyze whether this patient subset had a survival benefit associated with the study medication, if only patients in early disease stages were considered. This comparison showed a 14% superior 5-year survival estimate for patients treated with the combined decontamination medication, but this difference did not reach statistical significance due to the limited number of comparable patients in both study arms. Nevertheless, this difference provides a basis for a future study in this patient subset investigating the influence of anaerobic bacterial decontamination on survival as the primary study endpoint.
In conclusion, this open-label prospective randomized study provides evidence that antimicrobial chemotherapy targeted to intestinal anaerobic bacteria in marrow transplant recipients significantly reduces the severity of acute GVHD. The results of this study further support that the intestinal anaerobic bacterial microflora plays a role in the pathogenesis of acute GVHD after human marrow transplantation.
ACKNOWLEDGMENT
The authors are indebted to the nursing staff of the Department of Bone Marrow Transplantation at the University Hospital of Essen for their excellent patient care. The authors thank Angelika Hussel for her support in data aquisition and documentation. This work was made possible by the outstanding efforts of the technicians of the Department of Microbiology at the University of Essen.
This work is dedicated to Prof. Ulrich W. Schaefer at the occasion of his 60th birthday.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dietrich W. Beelen, MD, Department of Bone Marrow Transplantation, University Hospital of Essen, Hufelandstr. 55, 45122 Essen, Germany; e-mail: dietrich.beelen@uni-essen.de.