Abstract
Between 1970 and 1996, 333 patients with severe aplastic anemia underwent HLA-matched related marrow transplant after conditioning with cyclophosphamide (CY). Thirty-five percent of patients transplanted between 1970 and 1976 (group 1), 12% of those transplanted between 1977 and 1981 (group 2), and 9% of patients transplanted between 1982 and 1997 (group 3) had graft rejection. Graft rejection occurred later among group 3 patients (median, 180 days) than among those in groups 1 and 2 (medians, 28 and 47 days, respectively; P < .001 group 3 v 2). In group 3, 92% of rejecting patients underwent a second transplant, compared with 78% and 77% in groups 1 and 2, respectively. Group 1 patients received various conditioning regimens before second transplant, whereas most patients of groups 2 and 3 received CY combined with antithymocyte globulin (ATG). Graft-versus-host disease (GVHD) prophylaxis after second transplant consisted of methotrexate (MTX) for all group 1 and 2 patients, whereas group 3 patients received MTX combined with cyclosporine (CSP). Over the three time periods studied, first graft rejection decreased from 35% to 9%, and the proportion of rejecting patients undergoing second transplants increased from 77% to 92%. The 10-year probability of survival after second transplants increased from 5% to 83%. Multivariate analysis showed MTX/CSP GVHD prophylaxis to be a significant factor accounting for the increase in patient survival after second transplant.
© 1998 by The American Society of Hematology.
GIVEN THEIR NEAR NORMAL or normal T-cell responsiveness,1,2 patients with severe aplastic anemia (sAA) must be conditioned for allogeneic marrow transplants with immunosuppression to control T-cell mediated host-versus-graft (HVG) reactions and prevent graft rejection. Animal studies in the 1960s3-5 identified cyclophosphamide (CY) as a suitable conditioning agent that combined efficient immunosupression with acceptable toxicities when compared with total body irradiation (TBI). Indeed, since the first report of successful marrow allografts in patients with sAA in 1972,6 CY has remained the conditioning regimen of choice at the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) for those patients whose donors are HLA-matched family members.
With CY conditioning, sustained engraftment occurred in 90% of patients, and survival was 75%7 when patients were untransfused at the time of transplant. However, most patients referred for transplant were multiply transfused, and marrow graft rejection was seen in 30% to 70% of patients transplanted in the 1970s.8-13 Factors associated with rejection were transfusions before transplant (resulting in sensitization to minor histocompatibility antigens) and low marrow cell dose (<3 × 108/kg of body weight).14 The avoidance of transfusions, the use of γ-irradiated leukocyte depleted blood products, and the infusion of larger numbers of donor hematopoietic stem cells derived from marrow supplemented by peripheral blood buffy coat cells9,15 decreased rejection and improved survival, although the buffy coat cells increased the incidence of chronic graft-versus-host disease (GVHD). Also over time, more efficient immunosuppressive conditioning regimens were introduced such as the combinations of CY with antithymocyte globulin (ATG) or with total or partial body irradiation.16-19
Finally, improved postgrafting immunosuppression with the combination of methotrexate/cyclosporine (MTX/CSP) compared with monotherapy for GVHD prevention may also have decreased the rejection rate by suppressing HVG reactions, as shown in animal studies.20Owing to the changes in transfusion support and transplant techniques, the risk of rejection of first transplants in sAA patients has declined. Furthermore, for those patients undergoing a second transplant, the case fatality rate has decreased dramatically over the last three decades.
This report describes the changing incidence of graft rejection among 333 patients with sAA who received HLA-identical related marrow grafts at FHCRC since 1970 and analyzes the factors involved in increased survival after second transplants.
MATERIALS AND METHODS
From 1970 to 1996, 333 patients with sAA received HLA-identical related marrow transplants at the FHCRC after conditioning with CY. Patient data are shown in Table 1 for first transplants and Table 2 for second transplants. The patients were a median of 20.4 years old (range, 1.8 to 60.3 years old) at the time of first transplant and were 0.3 to 228 months (mean, 7.9 months; median, 1.9 months) from diagnosis. Diagnoses established at the referring institution were confirmed at this institution by review of outside marrow specimens and repeat marrow aspirates and biopsies. All patients had normal marrow cytogenetic findings. Marrow donors were HLA-A, HLA-B, and HLA-DR identical relatives. Mixed leukocyte culture was performed in all patients before the introduction of HLA-DR typing. One second graft was performed using peripheral blood stem cells (PBSCs); for all other grafts, the source of stem cells was marrow.
For purposes of the analysis, patients were divided into three groups reflecting time periods that coincided with changes in preparative regimens and GVHD prophylaxis used for first and second transplants. Accordingly, 81 patients transplanted from 1970 to 1976 constituted group 1, 104 patients transplanted from 1977 to 1981 were included in group 2, and group 3 consisted of 148 patients transplanted from 1982 to 1996.
Patients were referred to FHCRC after detailed consultation with their physicians and treated after outpatient and inpatient conferences that fully outlined the advantages and disadvantages of the transplant. Treatment protocols and consent forms were approved by the Institutional Review Board of the FHCRC.
First transplants.
Preparative regimen for first transplants included CY alone6 at 50 mg/kg intravenously (IV) for 4 consecutive days for 262 patients, CY plus procarbazine at 12.5 mg/kg IV on days −9, −7, and −5, alternating with rabbit ATG at 12 mg/kg subcutaneously on days −8, −6, and −4 for 19 patients,21 CY plus IV horse ATG (ATGAM; Upjohn Co, Kalamazoo, MI) at 30 mg/kg/dose, 12 hours after the first, second, and third dose of CY for 52 patients.19
GVHD prophylaxis was MTX only at 15 mg/m2 on day 1 followed by 10 mg/m2 on days 3, 6, and 11 and then once weekly until day 102 for 196 patients.6,21 Sixteen patients received CSP only at 1.5 mg/kg IV BID from day −1 through day 50, followed by progressive taper until day 180,22 whereas 121 patients received a short course of MTX (15 mg/m2 IV on day 1 and 10 mg/m2 on days 3, 6, and 11) combined with CSP in the dose schedule outlined above.23
Donor buffy coat infusions were administered to 125 patients in addition to the marrow as part of a prospective study.23
Assessment of hematopoietic engraftment and grading and treatment of acute and chronic GVHD were performed as previously described.24-26 Graft rejection was defined as either a failure to reach a granulocyte count greater than 1,000/μL for at least 3 consecutive days or by a progressive decrease in peripheral blood counts after initial engraftment, along with the redevelopment of an aplastic marrow. In addition, the disappearance of donor hematopoietic cells and reappearance of T lymphocytes of host origin were interpreted to represent graft rejection. In patients with sex-mismatched grafts, cytogenetics and more recently fluorescence in situ hybridization (FISH) analyses were used to monitor the graft. In the past, polymorphism in white blood cell enzymes served to assess grafts and graft rejection, whereas assays of DNA-based polymorphisms have been used since the mid-1980s.
Second transplants.
A variety of second transplant regimens were administered to group 1 patients, which included TBI (Table 2). CY/ATG became the standard regimen after 1977,27 except for 3 patients in group 3 who were either conditioned with IV CY at 120 mg/kg, followed by 1,200 cGy fractionated TBI (n = 2), or with 20 mg/kg/d methylprednisolone followed by taper through day 60 combined with IV anti-CD3 (BC3) monoclonal antibody at 0.4 mg/kg on day −1, 0.2 mg/kg on days 0 through 19, followed by progressive taper through day 26 (n = 1).28 Posttransplant GVHD prophylaxis consisted of long-term MTX (until day 102) for all patients of groups 1 and 2, whereas all group 3 patients received MTX/CSP.
Statistics.
Survival curves were calculated using Kaplan-Meier estimates.29 Incidence of graft rejection was calculated using cumulative incidence estimates where death before rejection was treated as a competing risk event.30 When survival or rejection after second transplant was shown, the time scale began at the time of second transplant. Comparisons of factors between patients transplanted in different time periods were performed using χ2 tests (or Fisher’s exact test, where appropriate), Wilcoxon’s rank sum test, or logrank test. Among patients receiving a second transplant between 1977 and 1996, the impact of factors on survival after the second transplant was evaluated using Cox proportional hazards regression.30 Variables examined included age at second transplant, gender, cell dose administered at transplant, length of interval between first and second transplant, date of second transplant (after 1982 v before 1982), and whether patients received MTX/CSP as GVHD prophylaxis versus MTX alone. Univariate models were initially examined. Because of the small number of patients in this study, full multivariate models with all variables could not be fit, although models including two variables at a time were evaluated.
RESULTS
First transplants.
Median patient ages at first transplant gradually increased over the three time periods (P = .04, group 3 v group 2). The time from diagnosis to transplant was the shortest between 1977 and 1981 (P < .0001, group 2 v group 1; P = .051, group 2 v group 3). Nearly all group 1 patients had multiple preceding transfusions, whereas almost one third of group 2 patients were untransfused (P = .26). In group 3, 78% of patients had prior transfusions. The marrow cell doses infused were comparable between the three groups, but all group 2 patients and approximately one third of the group 1 and 3 patients received additional buffy coat cell infusions from their donors.
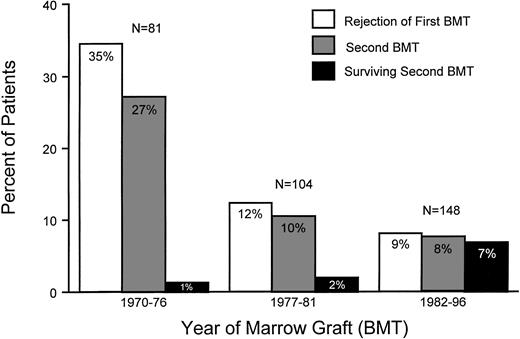
Overall, 53 patients (16%) rejected their first marrow graft. The highest rejection incidence (35%) was seen in group 1 patients, compared with 12% in group 2 (P < .001) and 9% in group 3 (P = .27, group 2 v group 3; Table 1 and Figs 1 and 2). Not only was rejection more frequent in earlier than in later patients, but rejection occurred significantly earlier in patients of groups 1 and 2 than in those of group 3 (P < .001; Fig 2). Specifically, the median time to rejection in group 1 patients was 28 days (range, 14 to 984 days), compared with 47 days (range, 14 to 215 days) in group 2 patients and 180 days (range, 22 to 583 days) in group 3 patients.
Incidences of rejection of first marrow graft, second grafts, and survival after second transplants among patients with aplastic anemia. Results are shown for three time periods: 1970 to 1976, 1977 to 1981, and 1982 to 1996.
Incidences of rejection of first marrow graft, second grafts, and survival after second transplants among patients with aplastic anemia. Results are shown for three time periods: 1970 to 1976, 1977 to 1981, and 1982 to 1996.
Probabilities of and times to graft rejection among 333 patients with sAA who underwent a first marrow graft after CY. Results are shown for three time periods: 1970 to 1976, 1977 to 1981, and 1982 to 1996.
Probabilities of and times to graft rejection among 333 patients with sAA who underwent a first marrow graft after CY. Results are shown for three time periods: 1970 to 1976, 1977 to 1981, and 1982 to 1996.
Second transplants.
The median patient ages at second transplant were comparable among groups 1 and 2 patients (P = .75), whereas group 3 patients were younger (median age, 10.6 v 20.3 years; groups 3 vgroup 2, P = .09).
In direct relation to the time to rejection, intertransplant intervals were the shortest in group 1 (median, 37 days) and group 2 patients (median, 57 days; P = .30, group 1 v 2) and the longest in group 3 patients (median, 226 days; P < .001, group 3v 2; Table 2 and Fig 2). The percentage of rejecting patients undergoing second transplants increased from about 77% (groups 1 and 2) to 92% (group 3; Fig 1). Those patients who did not receive second transplants either died before they had completed their second conditioning regimen (n = 3), showed autologous hematopoietic recovery (n = 4) before or during the second conditioning regimen, had developed secondary AML (n = 1), or, in yet another case, chose not to return to FHCRC for the recommended second transplant. The latter patient died of fungal infection several weeks after rejection was diagnosed. Furthermore, owing in part to the temporal proximity to the first transplant and in part to the greater intensity and toxicity of the conditioning regimens used in the earlier patients, approximately one third of group 1 patients died from toxicities or infections, too early after the second transplant for engraftment to be evaluated (Table 2). This percentage declined in the subsequent two time periods to 0% and 8.4%, respectively.
One third of group 1 and half of the group 2 patients rejected their second grafts. In group 1, all rejecting patients died from complications related to the prolonged pancytopenia. In group 2, 3 of 5 rejecting patients died from infections (1 from cardiomyopathy), and 1 recovered normal host hematopoiesis and is alive more than 15 years after second transplant. By comparison, only 3 patients of group 3 (25%) rejected the second graft, and all 3 are surviving with successful third grafts. Sustained second engraftment increased from 36% among group 1 patients to 50% and 67% in patients of groups 2 and 3, respectively (P = .04, group 3 v group 1;P = .68, group 2 v group 1).
Overall, only 1 of 6 engrafting patients in group 1 is alive, whereas the remainder died of infections between days 17 and 173. Similarly, only 1 of the 5 successfully engrafted group 2 patients is surviving; the remaining 4 patients died between 0.6 and 10 years, 3 from infectious complications associated with chronic GVHD and 1 from pulmonary hypertension. By comparison, all 8 engrafted patients in group 3 are alive, with follow-ups ranging from 2.8 to 12.2 years.
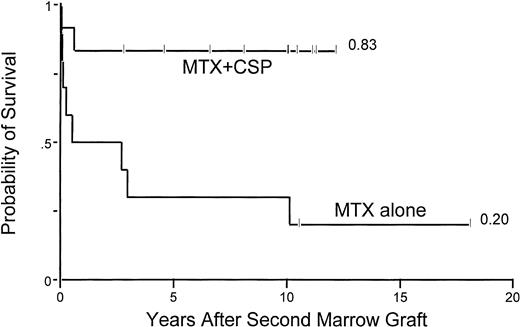
Acute grade 2-4 GVHD occurred in 2 of 6 (33%) group 1 patients with successful engraftment versus 5 of 5 (100%) group 2 patients (P = .06) and 2 of 8 (25%) group 3 patients (P= .02, group 2 v group 3). The use of MTX+CSP clearly contributed to the improved survival in group 3 patients, as shown in Table 3 and Fig3. The incidence of chronic GVHD among patients with sustained engraftment was 0% in group 1 patients, 80% in group 2 (P = .02, group 2 v group 1), and 50% in group 3 patients (P = .57, group 2 v group 3).
Probability of survival among 22 aplastic anemia patients who were conditioned with CY/ATG for their second marrow graft and received either MTX alone or the combination of MTX/CSP as postgrafting GVHD prophylaxis.
Probability of survival among 22 aplastic anemia patients who were conditioned with CY/ATG for their second marrow graft and received either MTX alone or the combination of MTX/CSP as postgrafting GVHD prophylaxis.
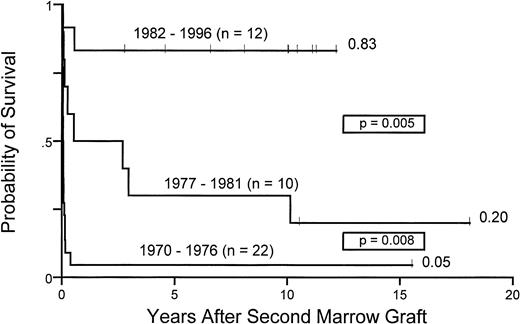
Figure 4 illustrates the improved survival after second transplants over the three time periods. Survivals increased from 5% (group 1) to 20% (group 2) and 83% (group 3), respectively.
Ten years probability of survival for 44 patients with aplastic anemia receiving second marrow transplants between 1970 and 1976, 1977 and 1981, and 1982 and 1996.
Ten years probability of survival for 44 patients with aplastic anemia receiving second marrow transplants between 1970 and 1976, 1977 and 1981, and 1982 and 1996.
Analyses of factors influencing survival in patients conditioned for second transplants with CY/ATG (groups 2 and 3).
Second transplant parameters included in the univariate analysis (Table 3A) showed that younger patient age (<18 years), longer intervals between first and second transplants, use of MTX+CSP GVHD prophylaxis, and not receiving buffy coat infusions were associated with improved survival after second transplant. Multivariate analysis showed the only significant factor to be MTX+CSP GVHD prophylaxis (Fig 3 and Table 3B).
DISCUSSION
Survivals of patients treated with marrow graft from HLA-identical family members at FHCRC have increased from 41% in the first half of the 1970s to 90% during the late 1980s and 1990s. At least three factors have contributed to the improvement in transplant outcome. One is the significant reduction in the incidence, severity, and mortality from acute GVHD. This has been accomplished through improved GVHD prophylaxis with the combination of MTX/CSP compared with either MTX or to CSP alone.23,31 The second factor is a decreased incidence of marrow graft rejection, from initially 35% to the current 9%. The third factor is increased survival after second transplants, which has increased from 5% to 83%. Accordingly, the overall mortality associated with graft rejection has decreased from 30% to 1.4 % over the 26-year study period. In fact, no patient has died due to graft rejection since 1988.32
Graft rejection in patients transplanted for aplastic anemia is usually the result of transfusion-induced sensitization to minor histocompatibility antigens, as documented in prospective animal studies33,34 and corroborated by analyses of results in transfused patients compared with those who were untransfused at the time of transplantation.7,35 Ninety-one percent of patients in group 1 had multiple transfusions; consequently, the rejection incidence was high. The progressive decrease in the rejection rates over the past 26 years has several reasons. Firstly, the percentage of untranfused patients increased from 9% in group 1 to 31% and 22% in groups 2 and 3, respectively. Secondly, increasing numbers of transfused patients received blood products from which antigen-presenting leukocytes have been removed (leukopoor) and which have been irradiated in vitro before administration. Both manipulations reduced the risk of graft rejection in animal studies.36-38Thirdly, most transfused patients in group 2 and many of those in group 3 (through June 1988) received donor buffy coat cell infusions in addition to the marrow graft, which were administered in an attempt at increasing the number of transplanted stem cells.39 Buffy coat cell infusions were discontinued in mid-1988 because of the unusually high incidence of chronic GVHD seen with this treatment modality.40 Fourthly, there have been improvements in the immunosuppressive quality of conditioning programs. For example, beginning in July 1988, all patients transplanted at FHCRC have been conditioned for their first graft with a combination of CY/ATG,19 which was known to be an effective conditioning program for second transplants.27 Other centers introduced radiation in the conditioning regimens, either in the form of TBI or partial body irradiation such as total lymphoid or thoraco-abdominal41 irradiation. Although effective in reducing rejection,17,42-44 the inclusion of irradiation may have increased the risks of GVHD,18,42 interstitial pneumonia,42,45 and secondary cancers, compared with CY-based regimens.46 Also, growth, development, and fertility may be impaired in irradiated patients.47-49 By comparison, no unusual short- or long-term side effects have been observed as yet with the CY/ATG regimen.32
Almost concurrent with the progressive decrease in rejection rates, survivals after second transplants increased. One important factor contributing to this success has been a significant delay in the time to rejection of the first graft from a median of 28 days among group 1 patients to 180 days in group 3 patients. In direct relation to the times to rejection, the intertransplant intervals increased. Patients undergoing second transplants after a longer intertransplant interval had recovered from toxicities associated with the first graft, and they were in much better clinical condition than those transplanted sooner after the first graft. Consequently, the patients were less likely to die from toxicities related to the second transplant regimen. The significantly delayed times to rejection in more recent patients are likely the result of the use of the short course of MTX combined with CSP for at least 6 months after transplant, compared with the earlier monotherapy with intermittent (once weekly) MTX for at most 3 months. Thus, although rejection rates in MTX/CSP treated patients were not significantly lessened, rejections that were observed occurred significantly later than in MTX-treated patients. In addition, MTX/CSP administered after second transplant reduced the severity of posttransplant related complications related to GVHD, thereby increasing patient survival. Other factors may also have been important, such as substituting the CY/ATG conditioning regimen for the previously used TBI containing regimens. However, with the small numbers of patients studied, the effect of the conditioning regimen was not found to be significant. Finally, gradual improvements in the quality of supportive care may also have contributed to the current success with second transplants.
ACKNOWLEDGMENT
The authors thank Harriet Childs and Bonnie Larson for their assistance in manuscript preparation and Deborah Monroe and Gary Schoch for their help in data collection.
Supported by grants from the Swiss National Foundation for Scientific Research, the Swiss League Against Cancer, the Fern Moffat Foundation of the Academic Society of the State of Vaud, Switzerland, and Grants No. HL36444 and CA15704 from the National Institutes of Health, DHHS (Bethesda, MD).
Address correspondence to Rainer Storb, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.