Abstract
Haptoglobin, a conserved plasma glycoprotein, forms very stable soluble complexes with free plasma hemoglobin. Hemoglobin binding by haptoglobin is thought to be important in the rapid hepatic clearance of hemoglobin from the plasma and in the inhibition of glomerular filtration of hemoglobin. To evaluate these functions,Haptoglobin knockout (−/−) mice were created. These mice were viable but had a small, significant reduction in postnatal viability. Contrary to popular belief, the lack of haptoglobin did not impair clearance of free plasma hemoglobin in −/− mice. Induction of severe hemolysis by phenylhydrazine caused extensive hemoglobin precipitation in the renal tubular cells of both −/− and +/+ mice, with death occurring in 55% of −/− mice and in 18% of +/+ mice. In general, phenylhydrazine-treated −/− mice suffered greater tissue damage, as evidenced by the induction of hepatic acute phase response resulting in increased plasma alpha 1-acid glycoprotein (AGP) levels. Among −/− and +/+ mice that survived, −/− mice tend to suffer greater oxidative damage and failed to repair or regenerate damaged renal tissues, as indicated by their higher plasma malonaldehyde (MDA) and 4-hydroxy-2(E)-nonenal (HNE) levels and lower mitotic indices in their kidneys, respectively. This study suggested that a physiologically important role of hemoglobin-haptoglobin complex formation is the amelioration of tissue damages by hemoglobin-driven lipid peroxidation.
© 1998 by The American Society of Hematology.
HAPTOGLOBIN (Hp) is a highly conserved plasma glycoprotein and is the major protein that binds free hemoglobin (Hb) with a high avidity (kd, ∼1 × 10−15mol/L).1,2 It is an acute-phase protein whose induction is evolutionally conserved, with the major inducers being interleukin-6 and related cytokines.3-7 The major site of synthesis is the liver, with moderate expression in the alveolar epithelium and adipocyte.8-10
It is generally accepted that the major function of Hp-Hb complex formation is the clearance of free Hb through endocytosis of the complex by specific receptors on liver parenchymal cells where the complex is rapidly degraded.11-13 Another important function of Hp-Hb complex formation is thought to be the retardation of passage of free Hb through the glomeruli into the renal tubular cells, resulting in renal damage.14 Excessive hemolysis or transfusion of Hb solution has been shown to result in Hp depletion and subsequent renal failure, in particular acute tubular necrosis. Hp has also been shown to be antioxidative,15angiogenic,16 and bacteriostatic.17 Hp may also be involved in the host defense responses to infection and inflammation (reviewed in Dobryszycka18).
Although Hp has been shown to have many biological activities, the physiological importance of these activities relative to similar activities performed by other molecules is not known. Of the many functions of Hp, the binding of Hb to form a Hb-Hp complex in a 1:1 stoichiometry appears to be the most important, as suggested by the extremely high avidity of Hp for Hb.1,2 However, the physiological significance of this complex formation remains an enigma. The intimate relationship of Hp with free plasma Hb is underlined by the universal use of Hp level as a clinical index of hemolysis.19-23 It has been shown that the Hb-Hp complex is rapidly removed from the blood through endocytosis by specific receptors localized on the liver parenchymal cells. The internalized complex is then rapidly degraded. These observations have led to a widely held belief that one function of Hp is to clear free plasma Hb. However, it has been previously reported that isolated liver parenchymal cells are capable of taking up free Hb at a faster rate than that of Hb-Hp complex, ie, rapid uptake of hemoglobin by liver parenchymal cells is not dependent on Hp-Hb complex formation.24 It has also been shown that haptoglobin binding has no effect on hepatic clearance and uptake of free hemoglobin from the plasma and that clearance of free Hb from the circulation is faster than that of Hb-Hp complex.25 26
To determine the physiological importance of Hb-Hp complex formation and the relative importance of Hp in general, Hp null mice were generated by homologous recombination in mouse embryonic stem (ES) cells. The relative efficiency and capacity for clearing plasma Hb were determined in mice lacking Hp and their wild-type littermates. The consequences of lacking Hp during severe hemolysis demonstrated the relevant role of Hp in exerting protection during tissue injury.
MATERIALS AND METHODS
Generation of Hp mutant mice.
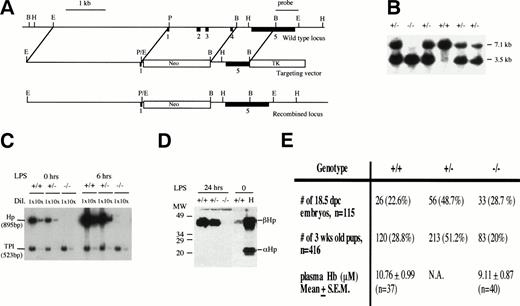
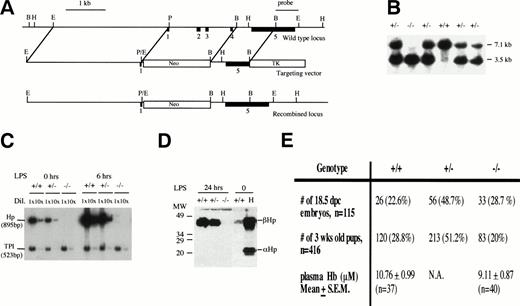
To design and construct the targeting vector, genomic DNA from theHp locus was isolated and mapped from a 129Sv genomic library (Stratagene, La Jolla, CA) using a mouse Hp cDNA probe. The targeting vector was constructed as described in Fig 1A. Gene targeting in ES cells and the generation of Hp mutant mice were performed as previously described.27 28 Briefly, the targeting vector was linearized with Sal I and electroporated into ES cells. The cells were selected with G418 and ganciclovir. About 20 doubly resistant ES cell clones were picked, expanded, DNA extracted, digested with EcoRI, and screened for homologous recombination at the Hp locus by Southern blot hybridization. In targeted ES cell clones, a 1.8-kb BamHI/HindIII single-copy probe derived from exon 5 and the 3′ flanking sequences that lies outside the region of homology detected a 3.5-kb EcoRI fragment instead of a 7.1-kb EcoRI wild-type fragment (Fig 1B). One targeted ES cell clone was injected into 3.5 days post-coitum (dpc) C57BL/6J blastocysts to generate several chimeras. The chimeras were mated with C57BL/6J females to produce heterozygous mice that were then intercrossed to produce mice homozygous for the mutation. Progenies from the heterozygous crosses were genotyped by Southern blot hybridization (Fig 1).
Creating an Hp null mouse. (A) Targeting strategy. Solid boxes denote exons 1 to 5. The vector in a 5′ to 3′ direction consists of a 3.1-kb EcoRI/Pst I fragment that includes sequences upstream of the hp gene, exon I and part of intron 1, a neomycin-resistant gene, a 1.3-kbBamHI fragment that includes part of intron 4 and exon 5, and a Herpes simplex viral thymidine kinase gene. Exons 2, 3, and 4 were deleted in a targeted allele. A 1.8-kbBamHI/HindIII fragment that includes part of exon 5 and the 3′ flanking sequences and lies outside the region of homology was identified as a single-copy probe and used in Southern blot analysis. (B) Southern blot analysis of progenies from Hp+/− inter-crosses using EcoRI digestion and the 1.8-kbBamHI/HindIII fragment as probe. The 7.1-kb fragment represents the wild-type allele and the 3.5-kb fragment represents the targeted allele. (C) Verification of Hp null phenotype by RT-PCR. RT-PCR using RNA from livers of +/+, +/−, and −/− mice at 0, 6, and 24 hours after LPS injection of 0.1 mg/10 g body weight. Briefly, 2 μg RNA from each genotype at various time points was reverse transcribed to cDNAs. The cDNAs were diluted 1× and 10× and amplified by PCR in the presence of 32P-dCTP usingHaptoglobin (hp) and triose phosphate isomerase (TPI) -specific primers to give 895- and 523-bp fragments, respectively. The RT-PCR products were separated on a 5% polyacrylamide gel, quantitated by phosphorimaging, and exposed to autoradiography. The Hpsignal was normalized against the TPI signal. No Hp mRNA was observed for −/− mice. There was about twice as much Hp mRNA in the liver of +/+ mice as there is in the +/− mice, and this ratio was maintained during induction by LPS. (D) Verification ofHp null phenotype by Western blot analysis. Mice were treated with LPS and 1 μL of plasma taken from each mouse 24 hours after treatment time was analyzed by Western blot analysis as described in Materials and Methods. There was no detectable Hp for −/− mice. Lane H represents the human Hp standard. (E) Genotype distribution of 3-week-old progenies from 10 different heterozygous breeder pairs and 18.5 dpc embryos from 13 different heterozygous crosses. For quantitation of plasma Hb, 5- to 6-week-old mice were lightly anesthetized and blood from the tail vein was collected in a heparinized capillary tube. The mean plasma Hb level in −/− mice was not significantly different from that in +/+ mice (P= .21).
Creating an Hp null mouse. (A) Targeting strategy. Solid boxes denote exons 1 to 5. The vector in a 5′ to 3′ direction consists of a 3.1-kb EcoRI/Pst I fragment that includes sequences upstream of the hp gene, exon I and part of intron 1, a neomycin-resistant gene, a 1.3-kbBamHI fragment that includes part of intron 4 and exon 5, and a Herpes simplex viral thymidine kinase gene. Exons 2, 3, and 4 were deleted in a targeted allele. A 1.8-kbBamHI/HindIII fragment that includes part of exon 5 and the 3′ flanking sequences and lies outside the region of homology was identified as a single-copy probe and used in Southern blot analysis. (B) Southern blot analysis of progenies from Hp+/− inter-crosses using EcoRI digestion and the 1.8-kbBamHI/HindIII fragment as probe. The 7.1-kb fragment represents the wild-type allele and the 3.5-kb fragment represents the targeted allele. (C) Verification of Hp null phenotype by RT-PCR. RT-PCR using RNA from livers of +/+, +/−, and −/− mice at 0, 6, and 24 hours after LPS injection of 0.1 mg/10 g body weight. Briefly, 2 μg RNA from each genotype at various time points was reverse transcribed to cDNAs. The cDNAs were diluted 1× and 10× and amplified by PCR in the presence of 32P-dCTP usingHaptoglobin (hp) and triose phosphate isomerase (TPI) -specific primers to give 895- and 523-bp fragments, respectively. The RT-PCR products were separated on a 5% polyacrylamide gel, quantitated by phosphorimaging, and exposed to autoradiography. The Hpsignal was normalized against the TPI signal. No Hp mRNA was observed for −/− mice. There was about twice as much Hp mRNA in the liver of +/+ mice as there is in the +/− mice, and this ratio was maintained during induction by LPS. (D) Verification ofHp null phenotype by Western blot analysis. Mice were treated with LPS and 1 μL of plasma taken from each mouse 24 hours after treatment time was analyzed by Western blot analysis as described in Materials and Methods. There was no detectable Hp for −/− mice. Lane H represents the human Hp standard. (E) Genotype distribution of 3-week-old progenies from 10 different heterozygous breeder pairs and 18.5 dpc embryos from 13 different heterozygous crosses. For quantitation of plasma Hb, 5- to 6-week-old mice were lightly anesthetized and blood from the tail vein was collected in a heparinized capillary tube. The mean plasma Hb level in −/− mice was not significantly different from that in +/+ mice (P= .21).
Verification of null phenotype in Hp−/− mice.
The null phenotype was verified by reverse transcription-polymerase chain reaction (RT-PCR) analysis of liver mRNA and Western blot analysis of plasma proteins from lipopolysaccharide (LPS)-treated mice. Briefly, 5- to 6-week-old mice were injected intraperitoneally (IP) with 0.1 mg LPS/10 g body weight. At 0, 6, and 24 hours, LPS-treated mice were killed and mRNA was extracted from the liver for analysis. RT-PCR was performed on the liver mRNA as previously described using Hp-specific primers 5′-AAA CGA CGA GAA GCA ATG GGT-3′ and 5′-GAA GGC AGG CAG ATA GGC ATG-3′ and triose phosphate isomerase (TPI)-specific primers 5′-CCC TGG CAT GAT CAA AGA CTT-3′ and 5′-GAT GGG CAG TGC TCA TTG TTT-3′ to give 895- and a 523-bp fragments, respectively.29 30 For Western blot analysis, the mice were bled from the tail vein 24 hours after injection. One microliter of plasma was separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto nitrocellulose, and probed with a goat antiserum against human Hp that also cross-reacts with mouse Hp (Sigma H5015; Sigma, St Louis, MO). The blot was then incubated sequentially with a biotinylated rabbit antigoat serum and a streptavidin-alkaline phosphatase before it was developed colorimetrically with 4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP).
125I-Hb clearance.
For 125I-iodination of mouse Hb, blood from a mouse was collected in EDTA by cardiac puncture. The red blood cells were purified from the white blood by ficoll-paque centrifugation, washed twice with phosphate-buffered saline (PBS), lysed in 4 vol of water, and centrifuged at 20,000g for 20 minutes at 4°C. The top half of the Hb was carefully removed for quantitation and iodination. Hb was quantitated using a kit by Sigma (S527A). The Hb was iodinated with Na125I using iodo-beads iodination reagent (Pierce, Rockville, IL) according to manufacturer’s protocol and purified using Sephadex G50. The concentration of 125I-Hb was adjusted to a final concentration of 1 mg/mL with cold Hb and was injected into the tail vein of anesthetized mice (5 to 6 weeks old) at 0.1 mg/10 g body weight. Mice were anesthetized with 0.1 mL/10 g body weight of a cocktail consisting of 1 part Hypnorm, 1 part Midazolom, and 2 parts distilled water. One minute after the injection was completed, the tip of the mouse tail was cut, a small aliquot of blood was collected in a heparinized capillary tube, and 10 μL of plasma was counted. This time point was designated 0 minutes. Thereafter, blood was collected at 5-minute intervals for 15 minutes, and the amount of radioactivity was calculated as a percentage of that at time 0.
Analysis of mice.
Anemia was induced in 5- to 6-week-old mice by IP injection (0.5 or 2 mg/10 g body weight) of freshly prepared phenylhydrazine. Phenylhydrazine hydrochloride (Sigma P6926) was dissolved in PBS at either 10 or 20 mg/mL and the pH was adjusted to pH 7.4 with NaOH. For free plasma hemolgobin quantitation, mice were lightly anesthetized with 0.05 mL/10 g body weight of a cocktail consisting of 1 part Hypnorm, 1 part Midazolom, and 2 parts distilled water. The tails were cut and blood was collected in a heparinized capillary tube. Care was taken to prevent hemolysis from pressure on tail during bleeding. Plasma Hb was quantitated using a kit (Sigma S527A). Measurement of plasma alpha 1-acid glycoprotein (AGP) by rocket immunoelectrophoresis was performed as previously described.31 For histological analysis, kidneys were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin-eosin. For malonaldehyde (MDA)/4-hydroxy-2(E)-nonenal (MDA/HNE) assays, plasma from blood collected in heparinized capillary tubes was assayed within 1 hour using a colorimetric assay kit for lipid peroxidation (Bioxytech LPO-586; Oxis International, Inc, Portland, OR) according to the manufacturer’s protocol. For the detection of Hb in tissue sections, standard immunohistochemistry using rabbit antimouse Hb serum (ICN catalogue no. 55447, lot no. 40433; ICN, Irvine, CA) as the primary antibody and horseradish peroxidase-conjugated swine antirabbit IgG antiserum as the secondary antibody.
Maximal hepatic acute-phase response was induced by two subdermal injections of 25 μL of sterile turpentine. Plasma was diluted 10-and 25-fold in PBS and 10-μL aliquots were analyzed by rocket immunoelectrophoresis31 using rabbit antimouse AGP antiserum. The area under the precipitation peak was integrated by using NIH image program version 1.61 (National Institutes of Health, Bethesda, MD). The arbitrary immunoelectrophoretic units were converted to milligrams per milliliter values by comparison with the values obtained with mouse acute-phase plasma standard, whose AGP standard had been calibrated based on purified mouse AGP protein.
RESULTS
Hp null mice: generation and viability.
The mouse Hp gene was inactivated by homologous recombination in mouse ES cells with a targeting vector that effectively replaced exons 2, 3, and 4 with a PGK-neo gene (Fig 1A).27 28 The targeting vector was electroporated into the E14 ES cell line. Two homologous recombinant clones were used to generate several chimeras. Only chimeras from one clone successfully transmitted the targeted allele to their progeny when crossed with C57BL/6J females (Fig 1B). Heterozygous mutant (+/−) mice were intercrossed to produce homozygous (−/−) mutant mice. Homozygous mutant mice were viable and fertile. RT-PCR and Western blot analysis demonstrated that both Hp mRNA and protein were absent in the liver and serum of the −/− mice, respectively (Fig 1D and E). The amount of Hp mRNA and protein in +/− mice was about half of that in +/+ mice, indicating that there was no compensation for the reduced gene dosage of Hp in +/− mice.
Although homozygous mutant mice were viable, their viability was compromised. The genotype distribution of 3-week-old offsprings from 12 different heterozygous breeder pairs was 28.8% +/+, 51.2% +/−, and 20% −/− (n= 416; Fig 1F). The number of mutant mice was significantly less than the expected 25% (P < .05). In contrast, the genotype distribution of 18.5 dpc embryos from 13 heterozygous crosses was 22.6% +/+, 48.7 % +/−, and 29% −/− (n = 115), indicating that the decrease in the number of 3-week-old −/− mice probably occurred after birth (Fig1E). The cause for their increased mortality is not known at this time. Therefore, the lack of Hp had a small but significant adverse effect on the postnatal viability of mice.
Hb clearance.
The average basal plasma Hb level in +/+ and −/− mice was not significantly different at 10.76 ± 1.0 μmol/L (n = 37) and 9.11 ± 0.9 μmol/L (n = 40), respectively (P = .21; Fig1F), suggesting that −/− mice were equally efficient in clearing free plasma Hb released from the normal turnover of red blood cells. To verify this, the relative efficiency of Hb clearance in +/+, +/−, and −/− mice was assessed by injecting a small amount of 125I-Hb (0.1 mg/10 g body weight) intravenously through the tail vein of these mice and measuring the loss of radioactivity from the plasma over time. There was a slight delay in the clearance of 125I-Hb from the plasma of −/− mice during the initial 10 minutes, but by 20 minutes, the level of residual 125I-Hb in the plasma was comparable to that of +/+ mice (Fig 2A). Therefore, −/− mice were able to clear Hb fairly efficiently through Hp-independent mechanisms, presumably by the liver, gut, and kidneys.32
(A) Rate of 125I-Hb clearance from the plasma. 125I-Hb (0.1 mg/10 g body weight; 7 × 105 cpm/μg Hb) was injected intravenously through the tail vein of anesthetized 5-to 6-week-old mice as described in the Materials and Methods. Two mice of each genotype were used and each data point represent the average from the two mice. (B and C) Rates of free plasma Hb accumulation and clearance during phenylhydrazine treatment. Different degrees of hemolysis were induced in 5- to 6-week-old mice by treating them with either two IP injections of 0.5 mg/10 g body weight spaced 8 hours apart on day 1 (B) or one IP injection of 2 mg/10 g body weight on day 1 (C). (Upper panels) plasma Hb level; (lower panels) mean hematocrit ± standard deviation (SD). A total of 7 −/− and 5 +/+ mice were used for (C), but two −/− mice died during the course of the experiment. In both studies (B and C), the levels of plasma Hb between +/+ and −/− mice at each time point were not significantly different by ANOVA (P = .35 and P = .89 for [B] and [C], respectively).
(A) Rate of 125I-Hb clearance from the plasma. 125I-Hb (0.1 mg/10 g body weight; 7 × 105 cpm/μg Hb) was injected intravenously through the tail vein of anesthetized 5-to 6-week-old mice as described in the Materials and Methods. Two mice of each genotype were used and each data point represent the average from the two mice. (B and C) Rates of free plasma Hb accumulation and clearance during phenylhydrazine treatment. Different degrees of hemolysis were induced in 5- to 6-week-old mice by treating them with either two IP injections of 0.5 mg/10 g body weight spaced 8 hours apart on day 1 (B) or one IP injection of 2 mg/10 g body weight on day 1 (C). (Upper panels) plasma Hb level; (lower panels) mean hematocrit ± standard deviation (SD). A total of 7 −/− and 5 +/+ mice were used for (C), but two −/− mice died during the course of the experiment. In both studies (B and C), the levels of plasma Hb between +/+ and −/− mice at each time point were not significantly different by ANOVA (P = .35 and P = .89 for [B] and [C], respectively).
To further assess the Hb clearing capacity of these mice during acute hemolysis, different degrees of hemolysis were induced in +/+ and −/− mice by either a single dose of phenylhydrazine (2 mg/10 g body weight via IP injection) on day 1 or two doses of phenylhydrazine (0.5 mg/10 g body weight via IP injection) spaced 8 hours apart on day 1.33-35 The severity of hemolysis was indicated by a decrease in the hematocrit level and an increase in the concentration of free plasma Hb that were proportional to the severity of hemolysis (Fig 2B and C).
The rates of free plasma Hb accumulation and subsequent clearance were not significantly different in +/+ and −/− mice under different degrees of hemolysis, suggesting that Hp was not essential for the physical clearance of free plasma Hb (Fig 2).
Susceptibility to phenylhydrazine-induced hemolysis.
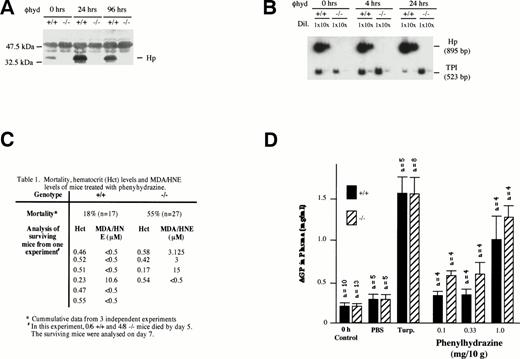
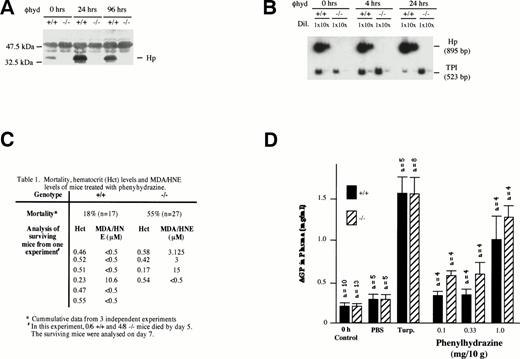
Despite the fact that plasma Hb clearance was not significantly different between −/− and +/+ mice during severe phenylhydrazine-induced hemolysis (2 mg/10 g body weight via IP injection; Fig 2C), −/− mice were more susceptible to severe hemolysis. More −/− (55%; n = 27) mice relative to +/+ (18%; n = 17) succumbed to the hemolysis within 5 days of the injection (Fig 3C). In both +/+ and −/− mice, severe hemolysis was evident on day 2 by a marked depression in hematocrit levels (<35%), dark brown coloration of both plasma, and urine indicating hemoglobinemia and hemoglobinuria, respectively. The mice generally began dying after 24 hours. Hp gene expression was induced in the liver of +/+ mice 4 hours after phenylhydrazine injection (Fig 3B). The level of plasma Hp was also elevated within 24 hours (Fig 3A).
Effects of phenylhydrazine treatment. (A) Plasma was extracted as described above from +/+ and −/− mice at 0, 24, and 96 hours after phenylhydrazine injections. One microliter of plasma was analyzed by Western blotting as described above. (B) RT-PCR using RNA from livers of +/+ and −/− mice at 0, 4, and 24 hours after phenylhydrazine injection of 2 mg/10 g body weight. RT-PCR was performed using Haptoglobin (Hp) and triose phosphate isomerase (TPI)-specific primers as described in Fig 1. The RT-PCR products were separated on a 5% polyacrylamide gel and exposed to autoradiography. The Hp signal was normalized against the TPI signal. Hp mRNA in livers of +/+ mice was elevated by 24 hours after phenylhydrazine injection. NoHp mRNA was observed in both livers and kidneys of −/− mice. (C) Mortality, hematocrit levels, and MDA/HNE levels of mice treated with phenylhydrazine. (D) Plasma AGP levels. Age-matched mice received either two subdermal injections of turpentine (Turp.), a single IP injection of PBS alone, or a single IP injection of the indicated concentrations of phenylhydrazine. Animals were bled before (0 hours, control) and 48 hours after injection. The concentration of AGP was determined by immunoelectrophoresis and the mean ± SD for the number of animals in each group shown.
Effects of phenylhydrazine treatment. (A) Plasma was extracted as described above from +/+ and −/− mice at 0, 24, and 96 hours after phenylhydrazine injections. One microliter of plasma was analyzed by Western blotting as described above. (B) RT-PCR using RNA from livers of +/+ and −/− mice at 0, 4, and 24 hours after phenylhydrazine injection of 2 mg/10 g body weight. RT-PCR was performed using Haptoglobin (Hp) and triose phosphate isomerase (TPI)-specific primers as described in Fig 1. The RT-PCR products were separated on a 5% polyacrylamide gel and exposed to autoradiography. The Hp signal was normalized against the TPI signal. Hp mRNA in livers of +/+ mice was elevated by 24 hours after phenylhydrazine injection. NoHp mRNA was observed in both livers and kidneys of −/− mice. (C) Mortality, hematocrit levels, and MDA/HNE levels of mice treated with phenylhydrazine. (D) Plasma AGP levels. Age-matched mice received either two subdermal injections of turpentine (Turp.), a single IP injection of PBS alone, or a single IP injection of the indicated concentrations of phenylhydrazine. Animals were bled before (0 hours, control) and 48 hours after injection. The concentration of AGP was determined by immunoelectrophoresis and the mean ± SD for the number of animals in each group shown.
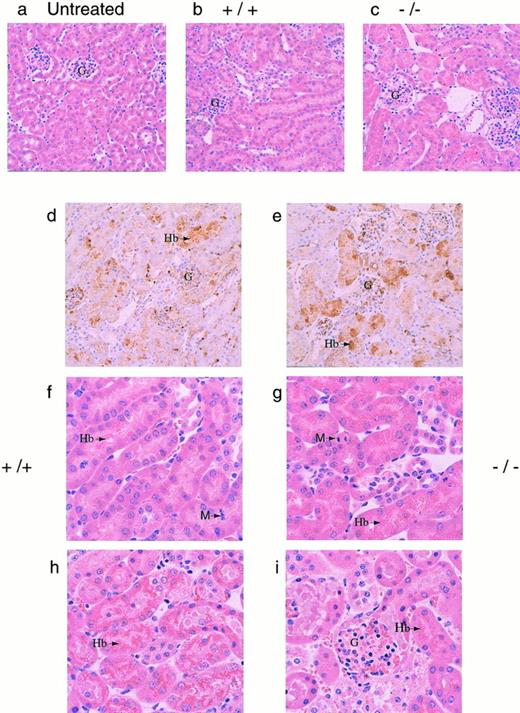
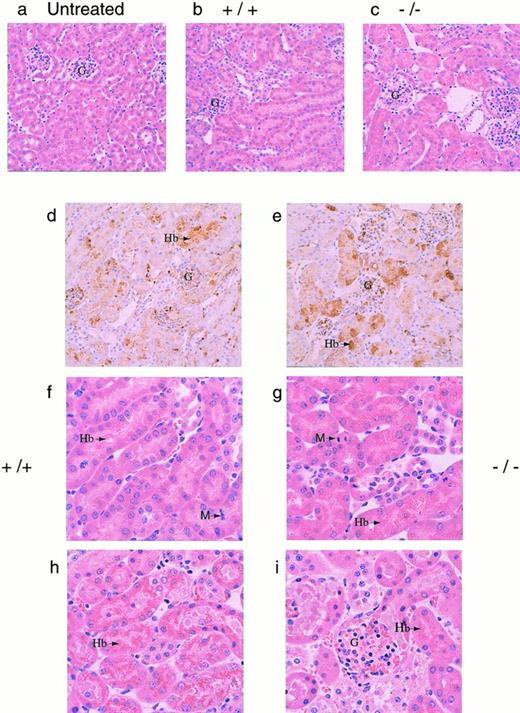
To determine a cause of death, several organs (kidneys, liver, lungs, heart, and spleen) were isolated from both +/+ and −/− mice 30 hours after phenylhydrazine injection. Histological analysis showed significant accumulation of hemoglobin precipitates in the renal tubules, but none of the other organs examined showed significant pathological changes (data not shown). Histological examination of kidneys from both +/+ and −/− mice that died during the course of phenylhydrazine treatment showed hydropic degeneration as evident by numerous cytoplasmic vacuoles and extensive Hb precipitation in the tubules in both +/+ and −/− mice (Fig 4). Based on these histological examinations, renal dysfunction was assumed to be a major factor in the mortality of these mice (Fig 4). The degree of Hb accumulation in the renal tubular cells was not discernibly different between +/+ and −/− mice at the gross morphological level. However, because it was not quantitated, it is possible that the degree of Hb accumulation in the renal tubular cells may be different between +/+ and −/− mice.
(a through e) Histological sections of kidneys. Kidneys from mice were fixed in 10% formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Representative sections as viewed under low power magnification (200×) from (a) a normal mouse (N), (b and c) phenylhydrazine-treated mice that survived, and (d and e) immunohistochemical analyses of kidney sections from phenylhydrazine-treated mice using a rabbit antiserum against mouse Hb. The presence of Hb was indicated by the brown precipitates. The glomeruli of phenylhydrazine-treated mice appeared relatively normal. Representative sections as viewed under high power magnification (400×). (f and g) Mice that survived the phenylhydrazine treatment; (h and i) mice that died during phenylhydrazine treatment. Hydropic degeneration, as evident by the numerous vacuoles, was observed. Arrows indicate Hb precipitates and M indicates metaphase cells found in histological sections of kidneys from phenylhydrazine-treated mice that survived. The mitotic index was calculated by randomly selecting 6 low power fields; within each low power field, the number of metaphase nuclei was counted against the total number of nuclei in 6 randomly chosen high power fields. Kidney sections from two mice of each genotype (+/+ and −/−) that survived the phenylhydrazine treatment were used to quantitate the mitotic index. The average mitotic index ± SD for +/+ mice was 18.8 ± 4.3 per 1,000 nuclei versus 4.78 ± 3.2 per 1,000 nuclei for −/− mice.
(a through e) Histological sections of kidneys. Kidneys from mice were fixed in 10% formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Representative sections as viewed under low power magnification (200×) from (a) a normal mouse (N), (b and c) phenylhydrazine-treated mice that survived, and (d and e) immunohistochemical analyses of kidney sections from phenylhydrazine-treated mice using a rabbit antiserum against mouse Hb. The presence of Hb was indicated by the brown precipitates. The glomeruli of phenylhydrazine-treated mice appeared relatively normal. Representative sections as viewed under high power magnification (400×). (f and g) Mice that survived the phenylhydrazine treatment; (h and i) mice that died during phenylhydrazine treatment. Hydropic degeneration, as evident by the numerous vacuoles, was observed. Arrows indicate Hb precipitates and M indicates metaphase cells found in histological sections of kidneys from phenylhydrazine-treated mice that survived. The mitotic index was calculated by randomly selecting 6 low power fields; within each low power field, the number of metaphase nuclei was counted against the total number of nuclei in 6 randomly chosen high power fields. Kidney sections from two mice of each genotype (+/+ and −/−) that survived the phenylhydrazine treatment were used to quantitate the mitotic index. The average mitotic index ± SD for +/+ mice was 18.8 ± 4.3 per 1,000 nuclei versus 4.78 ± 3.2 per 1,000 nuclei for −/− mice.
Mice that survived to day 7 generally recovered, with most +/+ (5/6) and −/− (3/4) mice regaining their normal hematocrit (Fig3C). Aside from the generally assumed function of Hp to clear Hb from the plasma, it has also been shown that, by binding Hb, Hp can inhibit the pro-oxidant activity of Hb.15,36-38 Therefore, the degree of lipid peroxidation in the plasma of surviving mice were measured using levels of MDA and HNE as indices.39 40 The MDA/HNE level in the plasma of normal untreated (+/+ and −/−) animals was less than 0.5 μmol/L in our assay. However, 3 of 4 −/− and 1 of 6 +/+ mice surviving a high dose of phenylhydrazine showed elevated MDA/HNE levels (Fig 3C). This result is consistent with the role of Hp inhibiting the peroxidative activity of Hb and indicates that the lack of Hp caused −/− mice to suffer greater oxidative damage. Furthermore, histological sections of kidneys from these surviving −/− mice showed less mitotic activity than +/+ mice, indicating that renal regeneration and repair were impaired in −/− mice (Fig 4f and g). Together, these data showed that, whereas both +/+ and −/− mice appeared to suffer severe renal damage, the high mortality rate, elevated MDA/HNE level, and lower mitotic index in −/− mice suggested that renal damage may be more severe in the −/− mice, resulting in poor recovery and regeneration of renal tissues.
Because it is unlikely that the protective action of Hp against severe phenylhydrazine-induced hemolysis was restricted to the kidneys, it is possible that −/− mice may also suffered a greater degree of tissue damage at multiple organ sites. Tissue damage would trigger a local inflammatory response that is followed by the recruitment of a systemic acute-phase reaction, leading to the hepatic induction of acute-phase plasma proteins. To assess if general tissue damage during phenylhydrazine-induced hemolysis was more severe in −/− mice than in +/+ mice, the magnitude of systemic acute-phase reaction as assayed by plasma concentration of AGP was measured. Subdermal tissue injury by low doses of turpentine resulted in a similar magnitude of AGP increase in both −/− mice and +/+ mice, indicating that inflammatory liver response was not compromised (Fig3D). When +/+ and −/− mice were treated with increasing doses of phenylhydrazine, plasma AGP levels in −/− mice were generally higher than that in PBS control-treated animals and in +/+ mice even at the lowest dose (0.1 mg/10 g body weight) of phenylhydrazine. The stronger acute-phase response indicates that −/− mice must have experienced greater tissue damages.
DISCUSSION
Hp has evolved in vertebrates as the major Hb binding protein. Its concentration in human plasma is inversely proportional to the extent of hemolysis and is used as a diagnostic marker for hemolytic processes.19-23 The strong noncovalent and irreversible binding of free plasma Hb by Hp and the presence of specific receptors on liver parenchymal cells that recognize and endocytose the Hp-Hb complex have led to a widely held belief that the major function of Hb binding by Hp is to target plasma Hb for rapid clearance and degradation in the liver.1,2,11-13 However, this belief is not consistent with previous observations that haptoglobin binding has no effect on hepatic clearance and uptake of free hemoglobin from the plasma, that the isolated liver parenchymal cells can take up free Hb at a faster rate than that of Hb-Hp complex, and that free Hb is cleared at a faster rate from the circulation than that of the Hb-Hp complex.24-26 These observations suggest that the clearing of plasma Hb as Hb-Hp complexes may not be a significant pathway, because the liver can potentially clear plasma Hb as free Hb more efficiently than as Hb-Hp complex. Results from our present studies with Hp knockout mice are consistent with this suggestion. The basal plasma Hb level in −/− mice was not significantly elevated, as would be expected if clearance of free plasma Hb by Hp was a significant pathway. Furthermore, the clearance of125I-labeled Hb and the rate of accumulation and clearance of plasma Hb during various degrees of hemolysis were also not significantly different between +/+ and −/− mice, suggesting that the efficiency and capacity for free plasma Hb clearance were not detectably compromised in −/− mice. Together, these data could not support the widely held belief that the major function of Hb binding by Hp is to target plasma Hb for rapid clearance and subsequent degradation in the liver. This study also demonstrated that, whereas Hp was not required for life and the clearance of free plasma Hb, its absence in mice caused a small but significant reduction in postnatal viability.
The strong avidity with which Hp binds Hb and the high conservation of the Hp gene across species suggest that Hb recognition and binding must be important physiological roles of Hp. These roles became evident by the increased susceptibility of −/− mice to tissue injury during phenylhydrazine-induced hemolysis. Histological analysis of organs from +/+ and −/− mice suggested that these mice, regardless of genotype, suffered extensive accumulation of Hb in the renal tubular cells with similar degree of hemoglobinemia. This is consistent with acute renal tubular necrosis (ATN) seen in patients with excessive hemolysis14 or during transfusions with stroma-free Hb.41 Yet, −/− mice appeared to suffered greater tissue damages, as evidenced by their significantly higher mortality rate of 55% versus 18% in +/+ mice and their greater acute-phase response during phenylhydrazine injection. This greater tissue damage probably resulted in the subsequent poorer renal regeneration and repair.
Clearly, these mice demonstrated that Hp confers some protective effects during hemolysis; this protection is likely to be mediated by the strong binding affinity of Hp for Hb. However, it is unlikely that the clearance of free plasma Hb is a significant factor in this protection, because the degree of hemoglobinemia was similar in both +/+ and −/− mice during hemolysis. Hb is a potent cytotoxic agent and its toxicity has been implicated in many diseases.42 In particular, Hb is a effective nephrotoxin.14,41 Hp is thought to reduce the nephrotoxicity of Hb by forming large macromolecular complexes with Hb and thus retarding the flow of free Hb through the glomeruli into the renal tubular cells. However, the extensive Hb precipitation in the kidney tubular cells of both +/+ and −/− mice suggests that retarding the flow of Hb through the glomeruli into the tubular cells may not be a significant factor in the protective effect by Hp in severe hemolysis. It has been shown that the Hb is a powerful pro-oxidant, particularly at acidic pH of 6.5, and that the binding of Hb by Hp inhibits this pro-oxidant activity.15,36-38 Consistent with this observation, plasma MDA and HNE levels, which were elevated in only a small fraction of phenylhydrazine-treated +/+ mice, were elevated in most of the −/− mice. Hb-driven lipid peroxidation may be most prominent in the renal tubular cells during severe hemolysis because of the high concentration of Hb and the general acidic milieu of the renal tubules that favors Hb-driven lipid peroxidation. This may explain the nephrotoxicity of Hb solutions observed during the development of Hb-based artificial blood.43
In conclusion, our study has shown that the likely and more important physiological function of the strong Hb-Hp binding is to inhibit Hb-stimulated lipid peroxidation and is not to facilitate the clearance of free plasma Hb by the liver for degradation, as is popularly believed. There are probably other functions associated with the strong Hb-Hp binding, but these remain to be defined. The Hp knockout mice would be useful in delineating some of these predicted functions and in characterizing their physiological importance. In vitro studies have already provided candidate functions that need to be considered. The well-documented vasoconstrictor activity of Hb solution that results from NO binding at the heme groups as well as the SH groups of Cysβ9344-51 is inhibited when Hb is bound by Hp.52 In −/− mice with severe hemolysis, the absence of Hp to bind Hb and inhibit its vasoconstrictor activity may result in renal vasoconstriction and ischemia, perhaps aggravating the effects of Hb precipitation in the renal tubular cells. The Hp knockout mice should prove useful in evaluating transfusions of cell-free Hb in which administration of Hb is often in excess of the Hb binding capacity of Hp in the plasma.
ACKNOWLEDGMENT
The authors thank Xiaolin Liang and Zaiqi Wu for technical support.
Supported by NUS RP 6600011 to S.-K.L. and National Institutes of Health Grant No. DK33886 to H.B.
Address reprint requests to Sai-Kiang Lim, PhD, Cardiovascular Research Institute, National University Medical Institutes, Blk MD11, 10 Kent Ridge Crescent, #02-01 CRC, Singapore 119260, Republic of Singapore; e-mail: nmilimsk@nus.edu.sg.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. (A) Rate of 125I-Hb clearance from the plasma. 125I-Hb (0.1 mg/10 g body weight; 7 × 105 cpm/μg Hb) was injected intravenously through the tail vein of anesthetized 5-to 6-week-old mice as described in the Materials and Methods. Two mice of each genotype were used and each data point represent the average from the two mice. (B and C) Rates of free plasma Hb accumulation and clearance during phenylhydrazine treatment. Different degrees of hemolysis were induced in 5- to 6-week-old mice by treating them with either two IP injections of 0.5 mg/10 g body weight spaced 8 hours apart on day 1 (B) or one IP injection of 2 mg/10 g body weight on day 1 (C). (Upper panels) plasma Hb level; (lower panels) mean hematocrit ± standard deviation (SD). A total of 7 −/− and 5 +/+ mice were used for (C), but two −/− mice died during the course of the experiment. In both studies (B and C), the levels of plasma Hb between +/+ and −/− mice at each time point were not significantly different by ANOVA (P = .35 and P = .89 for [B] and [C], respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.1870/4/m_blod41838002x.jpeg?Expires=1769381222&Signature=VeIAbEYdL3aEpR7GEzD7WAvb0xt1ajr1dJhEw7qnvbcm5y0DWEaSVlUi5r2Lm9Jg4CL6VMGsAq6NzPXZrhsNMgg555C0H21mGoHsuoNKozRj4QlYVDcrr4QwbKjD580JcWL6Xn3u9HWyydCnqbwOIlRHf0mNk1v16Pg5F5t4vzovZe~jcD2DdyGk28xBu~cwdhBYpfFzyGm6dWk7cyIXcXMandHuMB7Dsv93yb2nZOVQ24cCBBQu9Ygdz4KrzR1o28t7D-vvOb01-FY6e630YDkzqEY9x0-EztJMb4myJZFTY7ZMKa6RJ7kmXck1H~-lJKhDOpm4geINy~WaCGGRNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. (A) Rate of 125I-Hb clearance from the plasma. 125I-Hb (0.1 mg/10 g body weight; 7 × 105 cpm/μg Hb) was injected intravenously through the tail vein of anesthetized 5-to 6-week-old mice as described in the Materials and Methods. Two mice of each genotype were used and each data point represent the average from the two mice. (B and C) Rates of free plasma Hb accumulation and clearance during phenylhydrazine treatment. Different degrees of hemolysis were induced in 5- to 6-week-old mice by treating them with either two IP injections of 0.5 mg/10 g body weight spaced 8 hours apart on day 1 (B) or one IP injection of 2 mg/10 g body weight on day 1 (C). (Upper panels) plasma Hb level; (lower panels) mean hematocrit ± standard deviation (SD). A total of 7 −/− and 5 +/+ mice were used for (C), but two −/− mice died during the course of the experiment. In both studies (B and C), the levels of plasma Hb between +/+ and −/− mice at each time point were not significantly different by ANOVA (P = .35 and P = .89 for [B] and [C], respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.1870/4/m_blod41838002x.jpeg?Expires=1769381223&Signature=XIhLEvi36GUxY~RkzE3GeyxW~3X1UNjD~ACdfBx49M4gJtYaQbtmPdt4AJhleyKXdMeqhUYqelOT849LdXkVv7ZqX~h7C2uq9LNJONZTwaEjc21c82PfvFYHEyTbDbi8Wv8PNvwc2Wp80mcsXJzDNvJC77mCdudoS-MYYmsQ8XXl4vfGSMS3wk4dwR9HG9fSgcvMl~E0~VVE5Dotm6XbtVt4i3FJw8QAxbdPezzz0-eNi68g1t6w439l8cMcyY2MMguAwjefJEFeSgcSHmvaDF9iETd0RE0YOADJQJDkFc7d7A4mAsxrQILTNbx9jB3p5ZSp-mXmNuoYE1oChLtALg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)