Abstract
CD23 is a transmembrane protein expressed on the surface of B-lymphocytes that binds IgE, CD21, CD11b, and CD11c. High concentrations of soluble CD23 and L-selectin are found in the serum of patients with B-chronic lymphocytic leukemia (B-CLL). Because extracellular adenosine triphosphate (ATP) causes shedding of L-selectin via activation of P2Z/P2X7 receptors expressed on B-CLL lymphocytes, we studied the effect of ATP on shedding of CD23. ATP-induced shedding of CD23 at an initial rate of 12% of that for L-selectin, whereas the EC50 for ATP was identical (35 μmol/L) for shedding of both molecules. Furthermore, benzoylbenzoyl ATP also produced shedding of CD23 and L-selectin with the same agonist EC50 values for both (10 μmol/L). Inactivation of the P2Z/P2X7 receptor by preincubation with oxidized ATP abolished ATP-induced shedding of both molecules. Moreover, KN-62, the most potent inhibitor for the P2Z/P2X7 receptor, inhibited ATP-induced shedding of both CD23 and L-selectin with the same IC50 (12 nmol/L). Ro 31-9790, a membrane permeant zinc chelator that inhibits the phorbol-ester-stimulated shedding of L-selectin, also inhibited shedding of CD23 from B-CLL lymphocytes. However, the IC50 for this inhibition by Ro31-9790 was different for L-selectin and CD23 (83 v 6 μmol/L, respectively). Although L-selectin was completely shed by incubation of cells with phorbol-ester, CD23 was not lost under these conditions. The data show that extracellular ATP induces shedding of L-selectin and CD23 from B-CLL lymphocytes by an action mediated by the P2Z/P2X7 receptor. However, different membrane metalloproteases seem to mediate the shedding of L-selectin and CD23.
© 1998 by The American Society of Hematology.
THE CD23 ANTIGEN is a type II transmembrane protein of 45 kD that acts as a low-affinity receptor for IgE (FcεRII). This molecule is expressed on the surface of normal and malignant B cells, on monocytes, and to a lower extent on T cells, eosinophils, platelets, and a subset of dendritic cells.1CD23 is considered a B-cell activation marker because it is upregulated during B-cell stimulation by interleukin-4 (IL-4), IL-13, interaction with T cells, or contact with CD40 ligands.2 CD23 contains a C-type lectin domain which acts as the binding site for IgE, CD21, CD11b, and CD11c and is separated from the transmembrane domain by a coiled-coil stalk that facilitates dimer or trimerization.3Proteolytic cleavage of membrane-bound CD23 generates a soluble product of 37 kD that can be further degraded to 33, 25, and 12 kD.4
High levels of soluble CD23 and L-selectin are found in the serum of patients with B-chronic lymphocytic leukemia (B-CLL), suggesting that shedding of these molecules from malignant lymphocytes is a continuous process. Lower levels of soluble CD23 and L-selectin are also found in B-cell immunocytomas and low grade non-Hodgkin's lymphomas, whereas only tiny amounts of these soluble molecules can be detected in the sera of normal subjects. These factors suggest that soluble CD23 and L-selectin in B-CLL are derived from the circulating pool of lymphocytes and there is some evidence that soluble CD23 levels may reflect disease activity and be a prognostic indicator in B-CLL.5-10
Soluble forms of many membrane molecules have been described, although the mechanisms involved in their production are poorly characterized. Nonphysiological mediators such as phorbol esters are known to cause shedding of L-selectin by activating a zinc-containing membrane metalloprotease termed L-selectin sheddase.11 Recently, extracellular adenosine triphosphate (ATP) has been shown to mediate shedding of L-selectin from the surface of both normal and leukemic lymphocytes via activation of the P2Z purinoceptor of these cells.12 The gene for the human macrophage P2Z receptor has been cloned, and on the basis of extensive homology with the six described members of the P2X family of receptors, it is proposed that P2Z be termed P2X7.13,14 The P2Z receptor of lymphocytes shares many features with this cloned P2X7receptor, and it is likely that the two receptors are identical. First, the receptors are specific for the fully ionized ATP4−species and show a rank order of agonist potency of 2′- & 3′-0(4-benzoylbenzoyl) ATP (BzATP) ≫ ATP > 2-methylthio ATP > ATP-γ-S.15 Second, the receptor-operated channels are highly Ca2+ selective, although Ba2+ as well as fluorescent dyes such as ethidium+ or YoPro are also permeants.13,16 Finally, the receptors are inhibited partially by extracellular Na+ and totally by the isoquinoline sulphonamide derivative, KN-62, as well as by the irreversible inhibitor 2′, 3′ dialdehyde ATP (OxATP).15 17In this study we report that extracellular ATP induces shedding of CD23 from B-CLL lymphocytes. The characteristics of CD23 shedding have been compared with L-selectin shedding and the data suggest that both processes are mediated via the same P2Z/P2X7 receptor, although the receptor seems to activate different proteases.
MATERIALS AND METHODS
Materials.
ATP, BzATP, OxATP, and phorbol 12-myristate 13-acetate (PMA) were from Sigma Chemical Co (St Louis, MO). Ro 31-9790 (N-2-((2S)-[(hydroxycarbamoyl)methyl)-4-methylvaleryl]-N-1,3-dimethyl- L-valinamide) was a gift from Roche Products Ltd (Welwyn Garden City, UK). KN-62, 1-[N,O-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine was from Research Biochemicals, Inc (Natick, MA). Ficoll-Hypaque (density 1.077) was obtained from Pharmacia (Uppsala, Sweden). Fluorescein isothiocyanate (FITC)-labeled or purified mouse anti-human monoclonal antibodies (MoAbs) to L-selectin (CD62L) were either the DREG.55 clone (Bender MedSystems, Vienna, Austria) or the FMC46 clone (DAKO, Carpinteria, CA). FITC-, phycoerythrin (PE)-conjugated, and purified mouse anti-human MoAbs to CD23 were the Tü-1 clone from Bender MedSystems, the Leu-20 clone from Becton Dickson Co (San Jose, CA), and the MHM6 clone from DAKO. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG was obtained from DAKO. Enhanced chemiluminescence (ECL) kit was a product of Amersham (Buckinghamshire, UK). Flow Cytometry standard beads (Quantum 26) were from Flow Cytometry Standards Co (San Juan, CA).
Source of lymphocytes.
Peripheral blood lymphocytes with known P2Z/P2X7 responses were obtained from six patients with B-CLL and separated on Ficoll-Hypaque. Monocytes were depleted by plastic adherence for 1 hour. Cells were then washed once and resuspended in either Hepes-buffered NaCl medium (145 mmol/L NaCl; 5 mmol/L KCl; 10 mmol/L Hepes, pH 7.5; 5 mmol/L D-glucose; 0.1% bovine serum albumin [BSA]) or KCl medium (150 mmol/L KCl; 10 mmol/L Hepes, pH 7.5; 5 mmol/L D-glucose; 0.1% BSA). Cell morphology showed that greater than 99% of the mononuclear cells from B-CLL patients were small mature lymphocytes and immunophenotype showed 93.3 ± 8.8% of these lymphocytes typed as CD5+, CD19+, whereas 5.7 ± 2.5% typed as CD3+.
Immunofluorescence staining for L-selectin and CD23.
Aliquots of lymphocytes (1.0 × 106/mL) were incubated for up to 30 minutes in NaCl or KCl medium at 37°C with either ATP, BzATP, or PMA. In some experiments, lymphocytes were pretreated with 300 μmol/L OxATP for 1 hour. The inhibitors Ro 31-9790 or KN-62 were added 15 minutes before the addition of ATP or BzATP. The incubation was stopped by adding an equal volume of cold isotonic Mg2+buffer (20 mmol/L MgCl; 145 mmol/L NaCl; 5 mmol/L KCl; 10 mmol/L Hepes, pH 7.5). Cells were washed once and stained with FITC- or PE-conjugated anti–L-selectin or CD23 MoAbs. The mean channel fluorescence was collected on a FACScan flow cytometry (Becton Dickinson) in linear mode. The binding of Leu-20 MoAb to CD23 was independent of Ca2+ concentration in the medium.
Western blot for L-selectin and CD23.
Lymphocytes (1.0 × 108/mL) were treated with either 0.2 mmol/L BzATP or 100 nmol/L PMA at 37°C in NaCl medium without BSA plus proteinase inhibitors (5 mmol/L EDTA, 2 μg/mL aprotinin, 0.5 mmol/L phenylmethylsulfonylfluoride, 0.2 mg/mL leupeptin). After centrifugation and collection of supernates, the cells were washed once and lysed in 1% Triton X-100 containing 50 mmol/L Hepes, pH 7.0, plus proteinase inhibitors as above for 10 minutes. Cell lysates were clarified by centrifugation at 4000g for 15 minutes at 4°C. Western blotting was performed as previously described.18 Briefly, samples were separated on a 5% to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred onto nitrocellulose membranes. Membranes were blocked overnight in TTBS (20 mmol/L Tris; 150 mmol/L NaCl; 0.5% Tween 20, pH 7.5) containing 5% skim milk powder. Membranes were probed with antibodies as indicated. Bound antibodies were detected by HRP-conjugated goat anti-mouse IgG and enhanced chemiluminescence using an ECL kit (Amersham) according to manufacturer's instructions.
Statistics.
Mean values ± SEM are shown, whereas significance of differences was calculated using a Student's t-test.
RESULTS
Extracellular ATP causes loss of CD23.
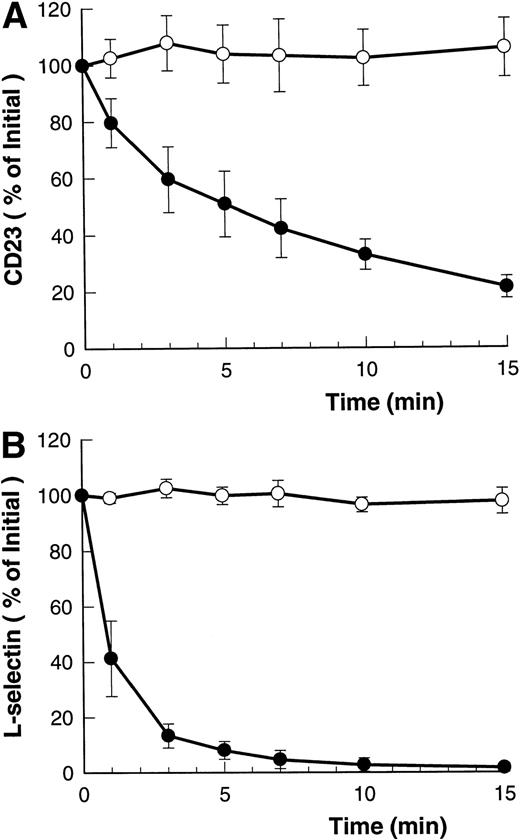
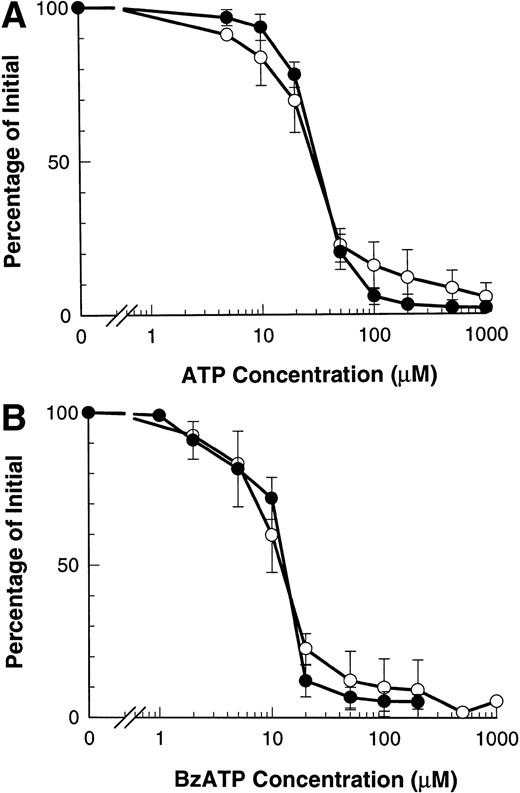
Figure 1A and B shows that both ATP and BzATP, a more potent agonist for the P2Z/P2X7 receptor, caused rapid shedding of L-selectin from the surface of B-CLL lymphocytes, as previously described.12 Both agonists for the P2Z/P2X7 receptor also caused disappearance of CD23 from the lymphocytes' surface (Fig 1A and B), but at a far slower rate than for L-selectin (7% v 60% per minute, respectively). Lymphocytes incubated in either isotonic NaCl or KCl showed a small but significant rate of spontaneous CD23 shedding with values of 1% to 3% over 5 minutes. In contrast to the above results, extracellular ATP did not alter the expression of CD44, CD45, CD47, CD49d, and HLA-DR on B-CLL lymphocytes (data not shown).
Time course of ATP- and BzATP-induced loss of CD23 and L-selectin. B-CLL lymphocytes were incubated with 500 μmol/L ATP (A) or 100 μmol/L BzATP (B) for the indicated times before labeling with anti-CD23 (○) or anti–L-selectin (•). Results are expressed as the mean percentage of initial expression ±SEM (n = 5).
Time course of ATP- and BzATP-induced loss of CD23 and L-selectin. B-CLL lymphocytes were incubated with 500 μmol/L ATP (A) or 100 μmol/L BzATP (B) for the indicated times before labeling with anti-CD23 (○) or anti–L-selectin (•). Results are expressed as the mean percentage of initial expression ±SEM (n = 5).
ATP releases soluble CD23.
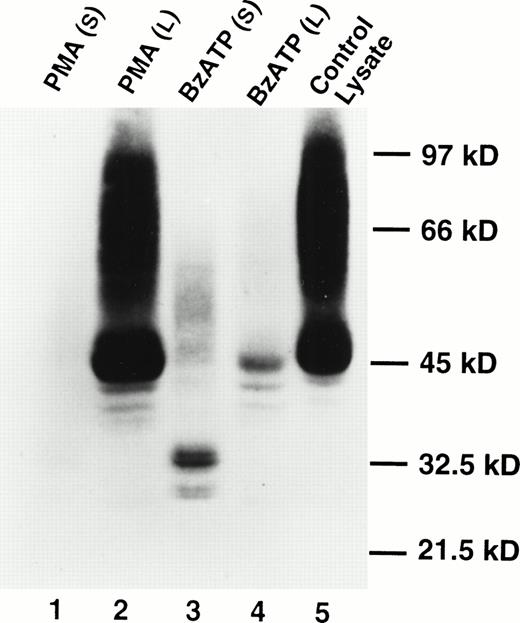
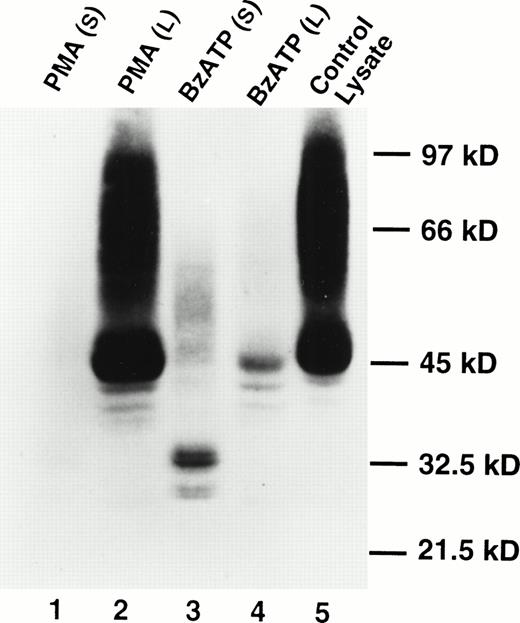
Western blotting showed the presence of soluble CD23 in the supernates of B-CLL cells treated with BzATP, but soluble CD23 was not detected in the supernates of cells treated with phorbol ester (Fig2). The appearance of soluble CD23 (Fig 2, lane 3) was associated with a loss of cell-associated CD23 in the Triton X-100 lysates (Fig 2, lane 4). Analysis of soluble CD23 in previous studies has identified multiple fragments of molecular sizes 37, 33, 25, and 12 kD.4 The principal band identified in Fig 2 was 33 kD with a lesser bands at 27 kD. The bands at 33 kD and 27 kD appeared as doublets. The significance of this is uncertain.
Appearance of soluble CD23 in the supernatant after lymphocyte exposure to BzATP but not PMA. Lymphocytes were treated with 100 nmol/L PMA (lanes 1 and 2) or 0.2 mmol/L BzATP (lanes 3 and 4) for 30 minutes. CD23 was detected in supernatants (lanes 1 and 3) or cell lysates (lanes 2 and 4) by Western blotting. Lane 5 is a cell lysate of untreated lymphocytes. No bands were detected using an irrelevant control antibody.
Appearance of soluble CD23 in the supernatant after lymphocyte exposure to BzATP but not PMA. Lymphocytes were treated with 100 nmol/L PMA (lanes 1 and 2) or 0.2 mmol/L BzATP (lanes 3 and 4) for 30 minutes. CD23 was detected in supernatants (lanes 1 and 3) or cell lysates (lanes 2 and 4) by Western blotting. Lane 5 is a cell lysate of untreated lymphocytes. No bands were detected using an irrelevant control antibody.
Agonist dose-response for CD23 shedding.
Our previous results show that ATP induces shedding of L-selectin by agonist occupancy of P2Z/P2X7 receptors.12 ATP induced the loss of CD23 from B-CLL lymphocytes with an EC50 of 29.7 ± 0.9 μmol/L, which did not differ significantly from the EC50 of 31.3 ± 1.2 μmol/L for ATP-induced loss of L-selectin (Fig3A). A similar result was obtained with BzATP which caused loss of CD23 with an EC50 of 11.3 ± 1.0 μmol/L and loss of L-selectin with an EC50 of 11.7 ± 0.5 μmol/L (P > .5, n = 3) (Fig 3B).
Dose response of ATP- and BzATP-induced shedding of CD23 and L-selectin. B-CLL lymphocytes were incubated with the indicated concentration of ATP (A) or BzATP (B) for 7 minutes before labeling with anti-CD23 (○) or for 1.5 minutes before labeling with anti–L-selectin (•). Results are expressed as mean percentage of initial expression ±SEM (n = 3).
Dose response of ATP- and BzATP-induced shedding of CD23 and L-selectin. B-CLL lymphocytes were incubated with the indicated concentration of ATP (A) or BzATP (B) for 7 minutes before labeling with anti-CD23 (○) or for 1.5 minutes before labeling with anti–L-selectin (•). Results are expressed as mean percentage of initial expression ±SEM (n = 3).
CD23 shedding is mediated via P2Z/P2X7 receptors.
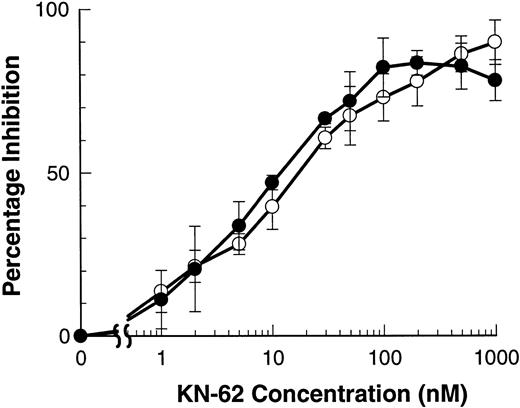
The isoquinolinesulfonamide derivative, KN-62, is the most potent inhibitor of the P2Z/P2X7 receptor described to date.17 The ATP-induced shedding of both L-selectin and CD23 were inhibited by KN-62 with an IC50 of 11.2 ± 1.2 and 16.0 ± 4.1 nmol/L that do not differ significantly (Fig4). Similar IC50 for KN-62 have been described for ATP-induced Ba2+ and ethidium+ influxes.17 Preincubation of lymphocytes with OxATP gives irreversible inhibition of the P2Z/P2X7 receptor of both murine macrophages and human lymphocytes.15,19 Lymphocytes were preincubated with or without OxATP (300 μmol/L for 60 minutes at 37°C) before incubation with BzATP (100 μmol/L) in NaCl-media. Figure5 shows that the shedding of both CD23 and L-selectin was completely abolished by OxATP preexposure. The downstream effects of ATP on lymphocytes are attenuated in high NaCl media that slows but does not abolish the shedding of L-selectin from B-CLL lymphocytes.12 Similarly, ATP-induced shedding of CD23 was slower in NaCl media than in KCl media (data not shown).
KN-62 inhibits ATP-induced shedding of CD23 and L-selectin. B-CLL lymphocytes were treated with the indicated concentrations of KN-62 before exposure to 500 μmol/L ATP (7 minutes for CD23 and 1.5 minutes for L-selectin measurements). Cells were then labeled with anti-CD23 (○) or anti–L-selectin (•) and the results expressed as the mean percentage of inhibition of shedding (difference of means not significant, n = 3).
KN-62 inhibits ATP-induced shedding of CD23 and L-selectin. B-CLL lymphocytes were treated with the indicated concentrations of KN-62 before exposure to 500 μmol/L ATP (7 minutes for CD23 and 1.5 minutes for L-selectin measurements). Cells were then labeled with anti-CD23 (○) or anti–L-selectin (•) and the results expressed as the mean percentage of inhibition of shedding (difference of means not significant, n = 3).
Inhibition of CD23 and L-selectin shedding by OxATP. B-CLL lymphocytes were treated with (○) or without (•) 300 μmol/L OxATP before exposure to 100 μmol/L BzATP. Expression of CD23 (A) and L-selectin (B) was measured and results expressed as the mean percentage of initial expression ±SEM (n = 3).
Inhibition of CD23 and L-selectin shedding by OxATP. B-CLL lymphocytes were treated with (○) or without (•) 300 μmol/L OxATP before exposure to 100 μmol/L BzATP. Expression of CD23 (A) and L-selectin (B) was measured and results expressed as the mean percentage of initial expression ±SEM (n = 3).
Phorbol esters induce shedding of L-selectin but not CD23.
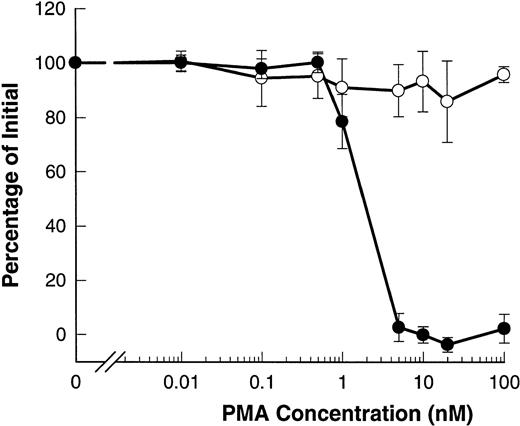
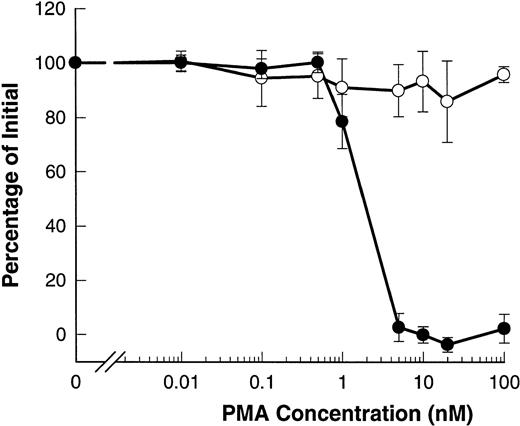
Phorbol esters such as PMA are known to induce shedding of L-selectin from B-CLL lymphocytes.20-22 Figure6 shows that PMA induced shedding of L-selectin with an EC50 of 2 nmol/L. However, PMA did not affect the expression of CD23 and even at doses as high as 100 nmol/L, no significant shedding of CD23 was observed.
PMA induces shedding of L-selectin but not CD23. B-CLL lymphocytes were exposed to the indicated concentrations of PMA for 15 minutes before measurement of CD23 (○) or for 1.5 minutes before measurement of L-selectin (•). Results are expressed as the mean percentage of initial expression ±SEM (n = 6).
PMA induces shedding of L-selectin but not CD23. B-CLL lymphocytes were exposed to the indicated concentrations of PMA for 15 minutes before measurement of CD23 (○) or for 1.5 minutes before measurement of L-selectin (•). Results are expressed as the mean percentage of initial expression ±SEM (n = 6).
The shedding of CD23 and L-selectin is inhibited by Ro 31-9790.
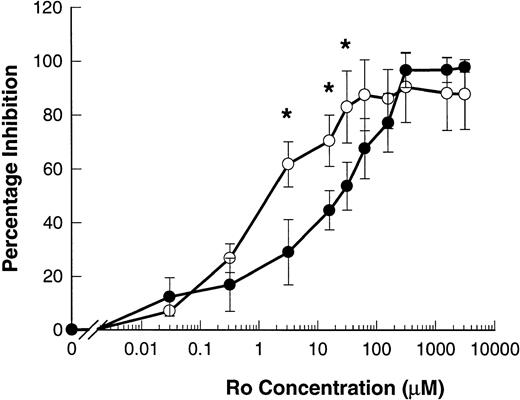
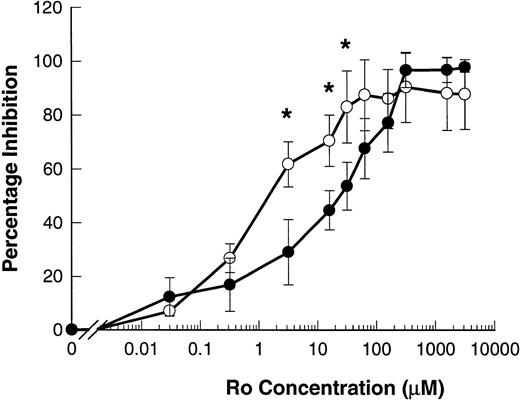
The hydroxamic acid derivative Ro 31-9790 is a lipophilic inhibitor of L-selectin sheddase, effective in the 2 to 10 μmol/L range in blocking phorbol-ester–induced shedding of L-selectin in human lymphocytes.11 23 Ro 31-9790 inhibited ATP-induced shedding of L-selectin with an IC50 of 82.5 ± 38.0 μmol/L. This inhibitor was even more potent in inhibiting ATP-induced shedding of CD23 with an IC50 of 5.7 ± 4.1 μmol/L (P = .013, n = 4) (Fig 7).
Ro 31-9790 inhibits ATP-induced shedding of CD23 and L-selectin. B-CLL lymphocytes were treated with the indicated concentrations of Ro 31-9790 before exposure to 500 μmol/L ATP for 7 minutes for CD23 and 1.5 minutes for L-selectin. CD23 (○) and L-selectin (•) expression was then determined and the results expressed as the mean percentage of inhibition of shedding ±SEM (n = 3). *, P < .05.
Ro 31-9790 inhibits ATP-induced shedding of CD23 and L-selectin. B-CLL lymphocytes were treated with the indicated concentrations of Ro 31-9790 before exposure to 500 μmol/L ATP for 7 minutes for CD23 and 1.5 minutes for L-selectin. CD23 (○) and L-selectin (•) expression was then determined and the results expressed as the mean percentage of inhibition of shedding ±SEM (n = 3). *, P < .05.
CD23 shedding is inhibited by extracellular Ca2+.
Extracellular ATP-induced shedding of L-selectin is independent of the presence or absence of Ca2+ in the medium,12 a finding we confirmed in the present study. However, ATP-induced loss of CD23 measured at 15 minutes was inhibited by 34% to 67% (n = 5) in the presence of 1 mmol/L Ca2+. The inhibitory effect of other divalent cations on the shedding of CD23 by ATP was studied. Mn2+ was the most potent inhibitor of CD23 loss and the order of inhibitory potency (at 1 mmol/L divalent concentration) was Mn2+ > Ca2+ = Mg2+ > Ba2+ (data not shown). Thus, the presence of Ca2+ and especially Mn2+ increased the resistance of CD23 to proteolytic shedding by approximately twofold.
DISCUSSION
Lymphocytes from patients with CLL express large numbers of receptors for extracellular ATP (P2Z/P2X7 receptors), and agonist competitive studies have shown only one class of purinoceptor, the P2Z/P2X7 receptor, is expressed on the surface of these cells.24 Activation of P2Z/P2X7 receptors induces multiple downstream effects, of which the best documented is the opening of an ionic channel that is selective for divalent cations.25,26 A second effect of ATP is to stimulate a phospholipase D, which on attachment to the plasma membrane, catalyzes hydrolysis of phosphatidylcholine to phosphatidic acid and choline.27,28 A third effect of ATP is to induce the shedding of L-selectin (CD62L),12 a C-type lectin that is involved in the adhesive interactions and rolling behavior of lymphocytes on endothelial cells.29 This study has identified another effect of ATP on B-CLL lymphocytes: the shedding of CD23 and appearance of soluble CD23 in the supernate. In contrast to the rapid shedding of L-selectin, the loss of CD23 induced by ATP was about one order of magnitude slower. Despite this difference in kinetics, several lines of evidence show that the ATP-induced loss of CD23, like L-selectin, is mediated by agonist activation of the P2Z/P2X7 receptor. Thus, CD23 and L-selectin showed identical dose-response curves for shedding by either ATP or the more potent agonist BzATP (Fig 3). Moreover, the potent P2Z/P2X7 receptor inhibitor, KN62, blocked the shedding of L-selectin and CD23 with the same IC50's (Fig 4), whereas pretreatment of lymphocytes with OxATP irreversibly inhibited the ability of P2Z/P2X7 agonists to induce shedding of either surface molecule.
There has been much recent interest in the nature of the protease responsible for the shedding of L-selectin. This enzyme does not cleave soluble substrates and is insensitive to a wide range of commonly used protease inhibitors, including diisopropylfluorophosphate, TIMP-1 (tissue inhibitor of metallo-proteinase-1), and phosphoramidon.11,23 The only known inhibitors are the hydrophobic hydroxamic acid derivatives such as Ro 31-9790 or KD-IX-73-4 which chelate zinc, whereas the water-soluble zinc-chelator o-phenanthroline is ineffective (unpublished observation). This requirement for lipid solubility of the inhibitor suggests that the enzyme that sheds CD23 contains zinc in a hydrophobic location, probably within the plasma membrane. How this enzyme is activated when agonist occupies the P2Z/P2X7 receptor is unknown. Members of the P2X receptor family have protein structures with two putative transmembrane segments linked by a long extracellular loop and intracellular N- and C-termini. The P2Z/P2X7 receptor is unusual in having a long C-terminal tail which not only confers unique permeability properties to large fluorescent cations,13 14but also has the potential for interactions with other membrane molecules such as proteases.
The initial rate of decrease of L-selectin immunoreactivity was 60% per minute but only 7% per minute for CD23 with either agonist (Fig1), indicating an 8.5-fold greater rate of shedding for L-selectin over CD23. It has been shown that phorbol ester-induced shedding of L-selectin from human leukocytes by L-selectin sheddase involves proteolytic cleavage at a K-S peptide bond.11,30 The structure of CD23 also includes three K-S bonds in its extracellular domain, although the most proximal cleavage site is 35 residues distal from the transmembrane domain compared with 10 residues for L-selectin. Assuming the catalytic site for proteolysis is membrane associated,11 and L-selectin sheddase cleaves both membrane proteins, then purely steric factors may account for the slower rate of CD23 shedding compared with L-selectin. However, recent data using site-directed mutagenesis has cast some doubt about the specificity of L-selectin sheddase for the K-S bond.31 Our data support a different hypothesis, namely that two different proteases are involved in the shedding of L-selectin and CD23. This latter possibility is supported by the failure of phorbol ester to shed CD23 (Fig 6) and the different sensitivity of shedding of L-selectin and CD23 to inhibition by Ro 31-9790 (Fig 7). Considering the wide range of membrane molecules that are known to be shed from the surface of leukocytes, it is likely that many different proteases are involved.32
A number of membrane molecules require extracellular Ca2+to maintain their tertiary or quaternary structure.33 Our data show that extracellular divalent ions inhibit the ATP-induced shedding of CD23. This result may reflect the stabilization of CD23 structure by Ca2+ such as shown for the resistance of the plasma cell membrane glycoprotein, PC-1, to proteolysis and thermal denaturation.33 However, the structure of the stalk region of CD23 (where enzymatic cleavage occurs) is an α-helical coiled-coil that does not contain a recognized Ca2+-binding domain and is unlikely to be stabilized by Ca2+ ions.3Thus, the mechanism by which Ca2+ inhibits ATP-induced CD23 shedding remains uncertain.
ATP-induced loss of CD23 immunoreactivity from the surface of B-CLL lymphocytes is associated with the appearance of soluble CD23 in the supernatant (Fig 2). Western analysis of the supernatant revealed two bands of molecular weights 33 and 27 kD, similar to those previously described in human plasma.34 High levels of both soluble CD23 and soluble L-selectin are found in the sera of patients with B-CLL, whereas levels of soluble CD23 have been related both to the tumor load and the clinical stage of this disease.5-10,21,37 Moreover, soluble CD23 has been proposed as a sensitive and specific marker for disease activity,6-8whereas in a complementary study high levels of cellular CD23 have been correlated with a favorable prognosis in B-CLL.37 Recent evidence suggests that L-selectin is cleaved from the surface of leukocytes within seconds during the process of rolling under hydrodynamic flow38 and it has been shown that hydroxamic acid-based inhibitors of zinc-dependent metalloproteases not only slow the rate of leukocyte rolling38 but also inhibit ATP-induced shedding of both L-selectin and CD23 (Fig 7). Because hydrodynamic shear causes release of ATP from endothelial cells,39 it is possible that the transient tethering that characterizes the rolling behavior of leukocytes may also provide the rapid signal for endothelial ATP release. This in turn could activate the P2Z/P2X7 receptor in the microenvironment of the tethered lymphocyte, stimulate the proteases that are coupled to this receptor, and generate both soluble L-selectin and soluble CD23.
ACKNOWLEDGMENT
We thank Tina Murphy for typing the manuscript and Roche Products Ltd for supplying Ro31-9790.
Supported by grants from the National Health & Medical Research Council of Australia and the New South Wales Cancer Council, Australia.
Address reprint requests to J.S. Wiley, MD, Sydney University Department of Medicine, Nepean Hospital, Penrith NSW 2750, Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.