Abstract
It has been shown that peripheral-blood mononuclear leukocytes (MNL) are responsible for transfusion-induced alloimmunization to donor major histocompatability complex (MHC) antigens. However, it is not known which subset of MNL is responsible for this immune response. Because elimination of class-II MHC antigen-positive passenger leukocytes effectively prolongs the survival of allografts, it has been hypothesized that class-II positive MNL are responsible for immunizing transfusion recipients to donor MHC antigens. To test this hypothesis, two different approaches were used. First, we compared the alloantigenicity of BALB/c mice (H-2d) peripheral blood MNL before and after depletion of class-II positive cells. CBA mice (H-2k) were used as transfusion recipients. Antibody development to donor class-I H-2 antigens was determined by flow cytometry and enzyme-linked immunoassay. After four weekly transfusions of MNL depleted for class-II positive cells, only 25% of recipient mice developed antibodies to donor H-2d antigens. In contrast, all mice transfused with control MNL became immunized. Second, we studied the alloantigenicity of peripheral MNL from C57BL/6 mice (H-2b) with homozygous deficiency of class-II MHC molecules in H-2 disparate recipient mice. After transfusions with class-II MHC molecule-deficient MNL, 0% of BALB/c, 40% of C57BR, and 25% of CBA-recipient mice developed antibodies to donor H-2b antigen. All control recipient mice were immunized. The antibody activities of the controls were also higher than those in the treatment group who became immunized. Thus, our study shows that class-II MHC antigen-positive MNL play a significant role in transfusion-induced alloimmunization to donor class-I MHC antigens. The results also support the hypothesis that direct antigen presentation by donor class-II positive MNL to the immune system of transfusion recipients is critical for the initiation of humoral immune response to donor MHC antigens.
ALLOIMMUNIZATION TO donor class-I major histocompatibility complex (MHC) antigens is one of the major immunological complications of blood component therapy. This complication often leads to platelet transfusion refractoriness or ineligibility of patients for transplantation of allografts. In view of the highly polymorphic nature of class-I MHC antigens and presence of these antigens in all components of blood,1 the development of antibodies to donor MHC antigens after transfusion should not be surprising. Nevertheless, class-I MHC antigens in soluble form or on isolated cell membranes have been found to be poorly immunogenic.2,3 This finding indicates that class-I MHC molecules by themselves are not effective immunogens, and intact donor leukocytes are responsible for transfusion-induced alloimmunization to MHC antigens. This conclusion is further supported by studies showing that removal or inactivation of leukocytes in platelet concentrates is effective for preventing transfusion-induced primary immunization to donor MHC antigens,4-8 even though leukocytes only contribute to less than 1% of total class-I MHC antigens in platelet concentrates.1 All these studies suggest that class-I MHC molecules cannot be efficiently processed by antigen presenting cells of transfusion recipients, and direct interaction between donor leukocytes and the recipient immune system plays a dominant role in transfusion-induced alloimmunization to donor MHC antigens.
It is therefore of interest to know which population of donor leukocytes is involved in alloimmunizing transfusion recipients. Results from such a study would provide a better understanding of the cellular mechanism by which the immune response is initiated in transfusion recipients. Previously, Faustman et al9reported that elimination of class-II positive passenger leukocytes was effective in prolonging the survival of pancreatic islet allografts in H-2 disparate recipient mice. Because class-II positive leukocytes including monocytes, dendritic cells, and B lymphocytes are known to provide important costimulatory signals for mounting successful immune responses10 and helper T cells can interact with antigen presenting cells carrying self or nonself class-II MHC molecules, it is likely that class-II positive donor leukocytes are responsible for direct initiation of transfusion-induced alloimmunization to class-I MHC antigens.
To test this hypothesis, we studied the effect of depleting class-II positive cells on the immunogenicity of donor leukocytes and investigated the alloantigenicity of peripheral blood leukocytes from mice with homozygous deficiency of class-II MHC molecules. The results are reported herein.
MATERIALS AND METHODS
Animals
Eight-week-old inbred CBA/CaH-T6J (CBA) and C57BR mice with H-2k MHC haplotype, BALB/cByJ (BALB/c) mice with H-2d haplotype, and C57BL/6 (BL/6) mice with H-2b haplotype were purchased from Jackson Laboratory (Bar Harbor, ME). Mice homozygous or heterozygous for the knockout of IA-b beta gene were generous gifts from Dr Christophe Benoist.11These class-II MHC antigen-deficient mice were bred into C57BL/6 background. All mice were housed in a temperature controlled room (25°C) with a 12-hour interval light/dark cycle and fed ad libitum. All animal studies were approved by the Institutional Animal Care and Use Committee.
Antibodies, Hybridomas, and Streptavidin Beads
Antimouse CD3, CD4, IA-b, IA/IE-d, and H-2b monoclonal antibodies (MoAbs) labeled with fluorescein isothiocyanate (FITC), antimouse CD8, and B220 MoAbs labeled with phycoerythrin, anti–IA-d, and anti–Thy-1.2 MoAbs labeled with biotin were purchased from PharMingen Corp (San Diego, CA). Hybridomas for anti–H-2b(HB51) and anti–H-2d (HB79) MoAbs were obtained from American Type Culture Collection (Rockville, MD). Anti–H-2b and H-2d MoAbs were purified from ascites as described.12 Streptavidin Dynal beads were purchased from Dynal Corp (Lake Success, NY).
Determination of Zygosity of Class-II MHC Molecule-Deficient Mice
The zygosity of class-II MHC molecule-deficient BL6 mice was determined by measuring numbers of CD4-positive and IA-b–positive cells in peripheral mononuclear leukocytes (MNL) using immunofluorescent flow cytometry, and by polymerase chain reaction (PCR) for the presence of neomycin resistance gene in genomic DNA. CD4-positive cells were severely deficient (<3% of peripheral MNL) and IA-b–positive MNL were absent in BL6 mice with homozygous deficiency of class-II MHC molecules. In contrast, there are 13% to 25% of CD4-positive T cells and 25% to 40% of IA-b–positive MNL in peripheral MNL of the heterozygous mice. Both the heterozygous and the homozygous mice were positive for neomycin resistance gene by PCR.
Preparation of Peripheral MNL
Peripheral MNL were prepared from freshly collected venous blood by differential and Ficoll-hypaque gradient centrifugation as described previously,7 and were suspended in phosphate-buffered saline (PBS). Concentrations of MNL were determined by hemacytometers after staining cells with propidium iodide.7
Depletion of Class-II MHC Antigen-Positive or Thy-1.2 Positive Leukocytes
Leukocytes positive for class-II MHC antigens were depleted by incubating 5×106 cells with 5 μg biotin anti–IA-d MoAbs (PharMingen) in 500 μL ice-cold PBS buffer containing 0.1% bovine serum albumin (BSA; [PBS-BSA]) for 30 minutes. The cells were washed twice with 2 mL of ice-cold PBS-BSA and resuspended in 0.5 mL PBS-BSA. The washed cells then were incubated with 5×107 M280 streptavidin magnetic beads (Dynal, Lake Success, NY) under constant rocking at 4°C for 20 minutes. The beads were washed extensively three times with PBS-BSA before use. The incubation was stopped by adding 0.5 mL PBS-BSA. After gentle mixing, free streptavidin beads and cells coated with the beads were removed magnetically. The cells that remained in the supernatant were harvested and washed twice with PBS and suspended in PBS for transfusion. The purity of the final cell preparation was assessed by immunofluorescent flow cytometry using FITC conjugate of anti-IA/IE-d MoAbs. For depletion of T cells, biotin anti–Thy-1.2 MoAbs and streptavidin beads were used by following the procedure as described above.
Transfusions of MNL
Each recipient mouse was transfused with donor leukocytes suspended in 100 μL PBS, once a week through a tail vein, under light anesthesia with inhalation of methoxyflurane (Pitman-Moore Inc, Mundelein, IL). Preimmune serum samples were prepared from venous blood collected through retro-orbital bleeding 1 or 2 days before the first transfusion. Thereafter, serum samples were collected after every two weekly transfusions of leukocytes and assayed for the presence of antibodies to donor MHC antigens. Numbers of donor leukocytes and transfusions given to each recipient mouse varied according to the experimental designs.
Immunofluorescent Flow Cytometry
The presence of antibodies to donor H-2 antigens was determined by immunofluorescent flow cytometry.7 Briefly, spleen MNL (1.5×105 cells) from mice of known H-2 phenotype were suspended in 20 μL PBS containing 0.1% sodium azide and 1% BSA (PBS-azide-BSA) and incubated with 10 μL serum at room temperature for 45 minutes. The cells were washed three times and stained with 30 μL of 40x diluted fluorescein-labeled goat antimouse IgG-Fc antibody (Sigma Corp, St Louis, MO) for 30 minutes. After three washes, cells were resuspended in 0.5 mL PBS-azide-BSA. The mean fluorescent intensities of 1×104 cells were measured in arbitrary units using a FACScan flow cytometer and Lysis-II software (Becton Dickinson, Palo Alto, CA). A pooled preimmune serum was used as a negative control and an immune serum known to contain antibodies against H-2 antigens on target cells was used as a positive control. Based on the result of our previous study,7 a serum sample that produced a mean fluorescent intensity greater than 1.5 times the mean fluorescent intensity of the pooled preimmune serum was considered positive for antibody to donor H-2 antigens.
Enzyme-Linked Immunoassay (EIA) for Detecting Antibodies to Class-I H-2 Antigens
To show the development of antibodies specific to class-I H-2 molecules in transfusion-recipient mice, serum samples were also assayed by EIA using purified class-I H-2 molecules as targets. For this assay, plastic wells of an immunoassay plate were coated with 50 μL of 0.75 μg/mL class-I H-2 molecules diluted in PBS containing 0.02% sodium azide. Class-I H-2 molecules were isolated from spleen cells as reported.12 After coating with class-I H-2b or H-2d molecules, each well was blocked with 1% BSA, and sequentially incubated with 5× diluted mouse serum, alkaline phosphatase conjugate of goat antimouse IgG antibody, and alkaline phosphatase substrate.12
RESULTS
Alloantigenicity of Peripheral MNL After Depletion of Class-II MHC Antigen-Positive Cells
Our first approach to investigate the role of class-II positive donor leukocytes in transfusion-induced alloimmunization was to study how depletion of class-II positive cells affected the immunogenicity of H-2 disparate donor MNL. Class-II positive leukocytes including B cells, monocytes, and dendritic cells were removed using biotin conjugate of anti–IA-d MoAb and streptavidin magnetic beads. According to immunofluorescent flow cytometric analyses, this purging method always removed more than 95% of class-II positive leukocytes (Table 1).
For this experiment, a well-established murine transfusion model was used.7 BALB/c mice were used as blood donors and CBA mice as transfusion recipients. After depletion of class-II MHC antigen-positive MNL, the remaining MNL were mostly T cells. Because recipient mice in the treatment group and the control group were transfused with different subsets of MNL, it was imperative to insure that both groups were exposed to the same amounts of donor class-I H-2d antigens. Our quantitative measurement of class-I H-2d antigens on donor MNL before and after purge of class-II positive cells showed that the amounts of class-I H-2d antigens on MNL after purge were reduced by 7% (Table1).
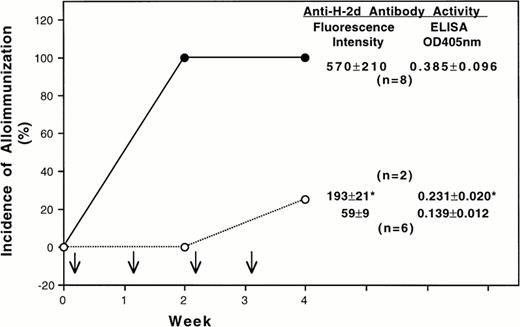
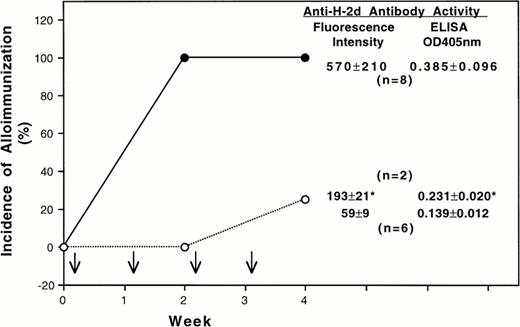
Because the quantitative difference of class-I H-2d antigens was small, the transfusion experiments were conducted by treating all recipient mice with the same number of leukocytes. After four weekly transfusions of 1×105 untreated donor BALB/c MNL, all CBA-recipient mice became immunized as expected (Fig 1). In contrast, only 25% of the CBA mice transfused with class-II positive cells depleted MNL developed antibodies to donor class-I H-2d antigens (Fig 1). The antibody activities of two immunized CBA mice in the treatment group were also lower than those of the control group. Similar results were obtained when the experiment was repeated.
Effect of depleting class-II MHC molecule positive leukocytes on transfusion-induced alloimmunization to donor class-I MHC antigens and leukocytes. In this experiment BALB/c (H-2d) and CBA (H-2k) mice were used as donors and recipients, respectively. Each recipient mouse was treated with four weekly transfusions (↓) of 1 x 105 donor peripheral mononuclear leukocytes with (○) or without (•) depletion of class-II positive cells. The average antibody activities ( mean ± SD) in the serum samples collected 1 week after the last transfusion are shown at the right side of each panel. The antibody activities were determined by using immunofluorescent flow cytometry and an enzyme-linked immunoassay. The fluorescent intensity and the OD405 nm for pooled preimmune sera were 52 and 0.128, respectively. (*) Average antibody activity of two CBA mice that became immunized after four transfusions of donor leukocytes depleted for class-II positive cells.
Effect of depleting class-II MHC molecule positive leukocytes on transfusion-induced alloimmunization to donor class-I MHC antigens and leukocytes. In this experiment BALB/c (H-2d) and CBA (H-2k) mice were used as donors and recipients, respectively. Each recipient mouse was treated with four weekly transfusions (↓) of 1 x 105 donor peripheral mononuclear leukocytes with (○) or without (•) depletion of class-II positive cells. The average antibody activities ( mean ± SD) in the serum samples collected 1 week after the last transfusion are shown at the right side of each panel. The antibody activities were determined by using immunofluorescent flow cytometry and an enzyme-linked immunoassay. The fluorescent intensity and the OD405 nm for pooled preimmune sera were 52 and 0.128, respectively. (*) Average antibody activity of two CBA mice that became immunized after four transfusions of donor leukocytes depleted for class-II positive cells.
Differential Leukocyte Counts in Peripheral Blood of Class-II MHC Molecule-Deficient Mice
The effects of class-II MHC deficiency on quantitative distribution of different subsets of lymphocytes in thymus, spleen, and lymph nodes have been reported.11 However, its effect on leukocytes in peripheral blood was not known. We therefore studied the distribution of different subsets of leukocytes in peripheral blood of C57BL/6 mice that were heterozygous or homozygous for the inactivation of IA-beta gene. Morphological examination and immunofluorescent flow cytometric analyses of peripheral blood showed that CD4+ cells were severely reduced in the homozygous mice and their B cells and monocytes were normal in number (Table 2). In heterozygous mice, class-II IA-b antigens were expressed at a normal level on leukocytes and the numbers of different subsets of leukocytes in these mice were the same as normal BL6 mice (Table 2). Because the heterozygous mice have normal phenotype, they were used as control donors. To avoid that different numbers of CD4+ and CD8+ cells in peripheral blood of homozygous and heterozygous donor mice complicated final interpretation of the transfusion study, we depleted T cells from peripheral MNL by biotin-labeled anti-Thy 1.2 MoAb and streptavidin magnetic beads. After magnetic purging, immunofluorescent flow cytometric analyses showed that greater than 95% of T cells were removed.
Immunogenicity of MNL from Class-II MHC Gene Knockout BL/6 Mice
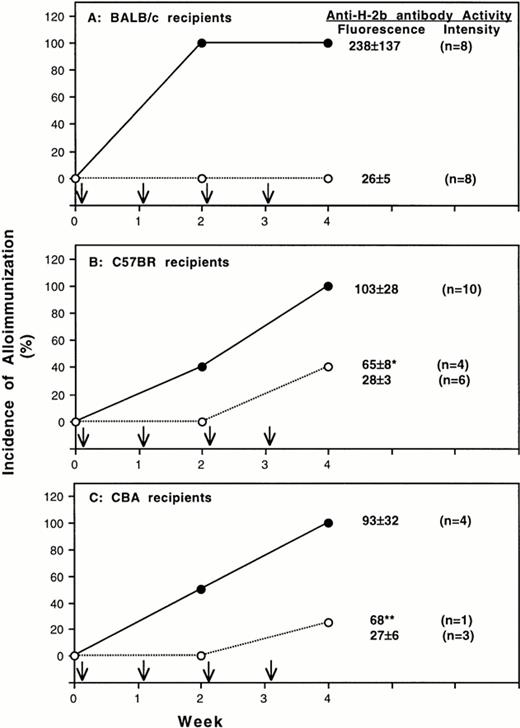
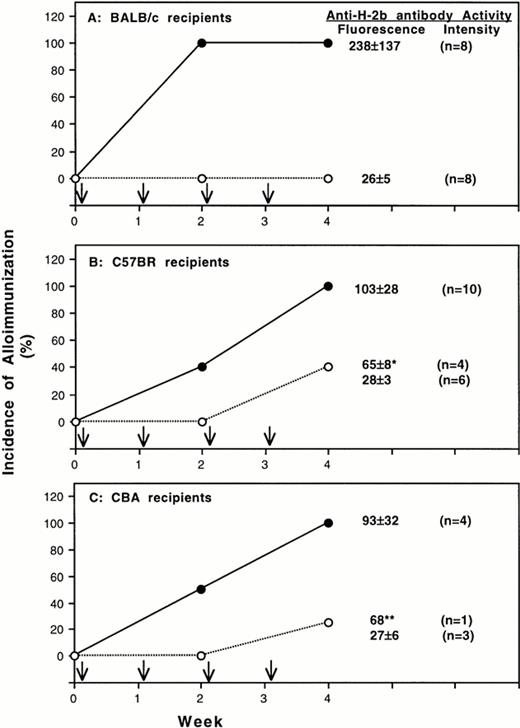
To determine the immunogenicity of MNL from class-II deficient mice, BALB/c, C57BR, and CBA mice were treated with four weekly transfusions of 25,000 T-cell–depleted peripheral MNL from BL/6 mice with heterozygous or homozygous deficiency of class-II MHC antigens. Serum samples were collected after every two weekly transfusions and were characterized for the development of antibodies to normal BL6 spleen MNL and class-I H-2b antigens. Results summarized in Fig 2A show that all BALB/c mice treated with heterozygous control MNL developed antibodies after two transfusions. In contrast, none of BALB/c (Fig 2A) became immunized after four transfusions of donor MNL with homozygous deficiency of class-II MHC molecules. Similar results were also obtained for C57BR and CBA-recipient mice. After four weekly transfusions, all mice in the control group became immunized. For those transfused with class-II deficient donor MNL, only 40% of C57BR- (Fig 2B) and 25% of CBA- (Fig 2C) recipient mice developed antibodies. As shown in Table 3, antibodies to donor class-I H-2b antigens were present in all immunized mice. Although some mice treated with homozygous-deficient MNL became immunized, the antibody activities in these mice were lower than those of the immunized control mice (Fig 2 and Table 3).
Alloantigenicity of peripheral blood mononuclear leukocytes with deficiency of class-II MHC molecule. Results of two separate experiments using BALB/c (H-2d) and C57BR (H-2k) mice as transfusion recipients are shown in panel A and B, respectively. Panel C shows the result from one experiment using CBA mice as recipients. Recipient mice were treated with weekly transfusion (↓) of 25,000 T-cell–depleted peripheral blood mononuclear leukocytes from BL6 donor mice with homozygous (○) or heterozygous (•) deficiency of MHC class-II molecules. The development of antibodies to donor leukocytes was determined by immunofluorescent flow cytometry. Spleen MNL from normal BL6 mice were used as targets. The average antibody activities (mean ± SD) in the serum samples collected 1 week after the last transfusion are shown at the right side of each panel. The fluorescent intensities of preimmune serum for BALB/c, C57 BR, and CBA mice were 24, 22, and 26, respectively. Four of 10 C57BR recipient mice treated with homozygous deficient MNL became immunized. The anti-H2b antibody activities of the four immunized C57 BR mice (*) were significantly lower than those of control mice (P = .02) (panel B). Only one CBA mice became immunized (**).
Alloantigenicity of peripheral blood mononuclear leukocytes with deficiency of class-II MHC molecule. Results of two separate experiments using BALB/c (H-2d) and C57BR (H-2k) mice as transfusion recipients are shown in panel A and B, respectively. Panel C shows the result from one experiment using CBA mice as recipients. Recipient mice were treated with weekly transfusion (↓) of 25,000 T-cell–depleted peripheral blood mononuclear leukocytes from BL6 donor mice with homozygous (○) or heterozygous (•) deficiency of MHC class-II molecules. The development of antibodies to donor leukocytes was determined by immunofluorescent flow cytometry. Spleen MNL from normal BL6 mice were used as targets. The average antibody activities (mean ± SD) in the serum samples collected 1 week after the last transfusion are shown at the right side of each panel. The fluorescent intensities of preimmune serum for BALB/c, C57 BR, and CBA mice were 24, 22, and 26, respectively. Four of 10 C57BR recipient mice treated with homozygous deficient MNL became immunized. The anti-H2b antibody activities of the four immunized C57 BR mice (*) were significantly lower than those of control mice (P = .02) (panel B). Only one CBA mice became immunized (**).
DISCUSSION
To show the role of class-II positive donor leukocytes in transfusion-induced alloimmunization to class-I MHC antigens, we first investigated how removal of class-II positive MNL affected the immunogenicity of donor MNL using a well-established BALB/c to CBA transfusion model.7,12 Although we were able to deplete 95% of class-II positive MNL, some class-II positive MNL remained. To minimize the potential interference by the remaining class-II positive MNL, we chose to transfuse each CBA recipient mouse with 1×105 BALB/c leukocytes. Based on our earlier study,7 this dose of undepleted MNL approached to a maximal but not supramaximal immunogenicity. The use of this dose of donor leukocytes not only provided a more stringent test system, but also allowed us to have sufficient sensitivity to detect any reduced antigenicity. To exclude the possibility that the reduced alloantigenicity in part resulted from exposure to less quantities of class-I MHC antigens on donor leukocytes after depletion of class-II positive cells, we measured the amount of class-I MHC antigens on donor leukocytes before and after depletion of class-II positive cells. We found that the difference was 7% and small (Table 1). According to our previous dose-response study,7 the immunogenicity of class-I H-2 molecules was slightly decreased after reducing donor leukocyte dose from 1×105 to 2.5×104 leukocytes. Because we transfused each mouse with 1×105MNL, 7% reduction of class-I MHC antigens on 1×105MNL should not have a significant impact on the final interpretation of our experiments.
In the present study, we also showed that peripheral MNL from BL/6 mice with homozygous deficiency of class-II MHC molecules have significantly reduced ability to immunize three different H-2 disparate strains of recipient mice (Fig 2 and Table 3). When BALB/c mice were used as recipients, none of them became immunized to donor class-I H-2b antigens (Fig 2A and Table 3). Although some C57BR and CBA recipient mice transfused with homozygous class-II deficient MNL became immunized, the antibody activities of the immunized mice were lower than those of the control group (Fig 2 and Table 3) and additional two weekly transfusions were needed to induce the antibody responses (Fig 2B and 2C). These results indicate that donor MNL with absence of class-II MHC molecules have reduced alloantigenicity
The presence of some immunogenicity of class-I MHC antigens in homozygous class-II deficient donor leukocytes is not unexpected. The absence of class-II MHC molecules deprives the direct interaction between donor antigen presenting leukocytes and H-2 disparate-recipient helper T cells for a vigorous humoral immune response. Nevertheless, significant numbers of class-I MHC antigens on MNL from homozygous class-II deficient donor mice still could be processed by antigen presenting cells of transfusion recipients and presented to their immune system. Despite poor efficiency of indirect processing and presentation of donor class-I MHC molecules by antigen presenting cells of recipients,3 this indirect antigen presentation pathway in C57BR and CBA recipient mice may be more efficient than in BALB/c mice. Consequently, class-I H-2b molecules on donor MNL and some contaminating platelets could have contributed to low levels of immunization as noted in some of our recipient mice (Fig 2B and 2C). Similarly, this indirect pathway could be responsible for the alloimmunization observed in some CBA mice treated with class-II depleted BALB/c MNL (Fig 1). These findings indicate that the indirect antigen presentation pathway is operational, albeit inefficient. This conclusion is consistent with an earlier report13 showing that donor class-I MHC antigens on platelets could be processed indirectly by antigen presenting cells of transfusion recipient mice to induce antibody development. Nevertheless, the indirect antigen presentation pathway is much less effective than the direct pathway as shown by transfusion experiments using leukocyte-depleted platelet concentrates to prevent alloimmunization to donor MHC antigens.4-8
In our study, we used BL6 mice with heterozygous deficiency of class-II MHC molecules as control donors. They were chosen because the heterozygous mice have normal immune function and phenotypes, and MNL from the heterozygous mice were as immunogenic as normal BL6 mice (data not shown). The use of heterozygous mice more importantly minimized genetic differences between control and class-II deficient donors. However, numbers of CD4-positive T cells are significantly reduced in mice with class-II homozygous deficiency. To insure that both groups of recipient mice were treated with the same subsets of donor leukocytes, we depleted all T cells in our transfusion study. We also chose to transfuse each recipient mouse weekly with 25,000 T-cell–depleted MNL. Because T cells account for 40% of peripheral blood MNL, this chosen dose is equivalent to cells obtained from 42,000 unselected peripheral blood MNL. According to our earlier study,7 this dose should have submaximal antigenicity. A submaximal immunogenic dose was chosen to minimize interference by indirect antigen presentation and by inadvertent activation of T-cell–depleted and class-II–deficient antigen presenting leukocytes, so that the test system can more clearly show the immunogenic role of class-II positive leukocytes.
Between the two experimental approaches used in our study there are major differences. These differences include different subsets (T cellsv antigen presenting B cells and monocytes) and different numbers (1×105v 2.5×104) of leukocytes that were used for transfusion. Because two different subsets of leukocytes have very different functions and immunogenicity, it is unlikely that we can confirm varying efficiencies of indirect antigen presentation between different strains of recipient mice by repeating the class-II depletion transfusion experiment using the same strain combinations in the class-II knockout transfusion experiments. For this reason, we did not use the same strain combinations to repeat the class-II depletion transfusion experiment.
In conclusion, the results of our study have shown the importance of class-II positive leukocytes in transfusion-induced alloimmunization to donor class-I MHC antigens and supported that direct antigen presentation by donor class-II positive leukocytes is the predominant pathway for alloimmunization to MHC antigens.
ACKNOWLEDGMENT
We thank Sandra Donahue for her excellent technical assistance.
Supported by a grant from the National Institutes of Health (HL58809-01).
Address correspondence to K.J. Kao, MD, Department of Pathology, Immunology, and Laboratory Medicine, Box 100275, University of Florida, Gainesville, FL 32610; email: kjkao@ufl.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.