Abstract
Rapamycin is an immunosuppressant that effectively controls various immune responses; however, its action in the signal transduction of lymphocytes has remained largely unknown. We show here that a phosphoprotein encoded by mouse α4 (mα4) gene transmitting a signal through B-cell antigen receptor (BCR) is associated with the catalytic subunit of protein phosphatase 2A (PP2Ac). The middle region of α4, consisting of 109 amino acids (94-202), associates directly with PP2Ac, irrespective of any other accessory molecule. Rapamycin treatment disrupts the association of PP2Ac/α4 in parallel with the inhibitory effect of lymphoid cell proliferation. The effect of rapamycin was inhibited with an excess amount of FK506 that potentially completes the binding to FKBP. Rapamycin treatment also suppresses the phosphatase activity of cells measured by in vitro phosphatase assay. Introduction of the mα4 cDNA into Jurkat cells or the increased association of PP2Ac/α4 by the culture with low serum concentration confers cells with rapamycin resistance. Moreover, glutathione S-transferase (GST)-α4 augments the PP2A activity upon myelin basic protein (MBP) and histone in the in vitro assay. These results suggest that α4 acts as a positive regulator of PP2A and as a new target of rapamycin in the activation of lymphocytes.
CROSS-LINKING OF B-cell antigen receptor (BCR) induces activation of multiple signal transduction pathways associated with the BCR-complex.1-6 However, the signal affected by the Immunosuppressant rapamycin has largely remained unsolved. Two analogous immunosuppressants, cyclosporin A (CsA) and FK506, bind to cyclophilin and FKBP12, respectively,7 both of which inhibit the activity of calcineurin (protein phosphatase 2B),8 a phosphatase that regulates NF-AT activity involved in T-cell receptor signal transduction.9 Rapamycin of a similar structure to FK506 associates with the same FKBP, but the action thereafter is likely to be different.10,11 Rapamycin is shown to inhibit the growth signal mediated through the interleukin-2 receptor (IL-2R) and the downstream molecule involved in the rapamycin-sensitive pathway is named TOR (target of rapamycin) in yeast and mammalian cells.12-16 The rapamycin/FKBP complex interacts with TOR, subsequently inhibiting the activity of S6 kinase (S6K) that promotes cell cycle progression at G1 to S phase.17 Interaction of rapamycin/FKBP also inhibits the downregulation of p27 kip, which negatively regulates cell cycle progression.18 Despite this evidence, it has not been determined whether the molecules are interacting directly in the downstream signals through rapamycin/FKBP/TOR-sensitive pathways.
In a search of molecules involved in the BCR-mediated signal transduction, we identified a component associated with Ig-α (CD79a, MB-1) and isolated a candidate cDNA clone (α4) using a monoclonal antibody (Ab).19, 20 The α4 is phosphorylated in vivo by phorbol 12-myristate 13-acetate, and BCR cross-linking induces a transient association of α4 with a tyrosine-phosphorylated molecule, indicating the involvement of the α4 in the BCR-mediated signal transduction.20 The molecule, now designated as Ig receptor binding protein 1 (α4), has several phosphorylatable sites by protein kinase C and casein kinase 2 (CK2) and a proline-rich sequence that is a possible interaction site with SH3 motif. Recently, a yeast homologue of α4 named Tap42 was cloned, and it was shown that nutrient growth signal induces association of Tap42 with protein phosphatase 2A (PP2A) and the related phosphatase SIT4.21 It was also shown that rapamycin inhibits the growth of yeast by dissociating Tap42 from PP2A/SIT4.
PP2A is a major intracellular serine/threonine phosphatase and plays pivotal roles in the control of cell cycle, proliferation, viral transformation, and metabolic pathways.22-24 PP2A represents complex structures in various cells as a family of holoenzymes consisting of a common core dimer of a 39-kD catalytic C subunit and a 65-kD A subunit associated with various regulatory B subunits. A and C subunits are expressed ubiquitously, but B subunits are expressed in a tissue-specific and developmentally regulated manner.25-27 These regulatory subunits can modify PP2A enzyme activity, substrate specificity, or subcellular localization of the enzyme.23,24,26,27 Recent studies further reported different types of binding molecules to the catalytic subunit of PP2A (PP2Ac). These types include eRF1,28 Hox11,29and CK2,30 which showed binding to PP2Ac in cells in addition to their unique functions such as the protein synthesis, the nuclear transcription factor activity, or the kinase activity of several cellular protein molecules. These molecules presumably participate in cell proliferation: eRF1 targets the PP2A to polysome without affecting enzyme activity, Hox11 inhibits the enzyme activity of PP2A and induces M phase of the cell cycle, and, conversely, CK2 augments PP2Ac activity by phosphorylating PP2Ac.
We show here that human Igbp1/α4 (hα4) is directly associated with PP2Ac in human lymphoid cell lines through the middle portion of α4 protein. Rapamycin disrupts the PP2Ac/α4 interaction in rapamycin-sensitive Jurkat cells but not in rapamycin-resistant Raji cells. We demonstrate that the introduction of mouse α4 (mα4) into Jurkat cells conferred rapamycin resistance, indicating that PP2A/α4 acts as a new target of rapamycin. Furthermore, α4 augments the enzyme activity of PP2A both in vitro and in vivo and rapamycin inhibits the PP2A activity in Jurkat cells by disrupting the PP2A/α4 association.
MATERIALS AND METHODS
Reagents and Abs.
Rapamycin was purchased from Wako Chemicals (Osaka, Japan). FK506 was purchased from Fujisawa Pharmaceutical Co (Osaka, Japan). Anti-PP2Ac Ab was purchased from Upstate Biotechnology Inc (Lake Placid, NY). Anti-mα4 Ab was prepared and characterized previously.20Anti-hα4 serum was prepared by immunizing rabbits with the GST-hα4. The serum purified by the GST-hα4 affinity column was used as anti-hα4.
cDNA construct and fusion proteins.
Human PP2Ac and hα4 cDNAs were prepared by reverse transcriptase with oligo dT primer from mRNA of human B-lymphoid cell line RPMI8866 and the subsequent polymerase chain reaction (PCR) reaction. The primers for the amplification were 5′-GGATCCTCATGGACGAGAAGGTGTTC-3′ and 5′-GGATCCCAGGAAGTAGTCTGGGGTAC-3′ for PP2Ac31 and 5′-GGATCCAGATGGCTGCTGAGGACGAG-3′ and 5′-GGATCCGCCCATGTTCTGTCGGTTCC-3′ for α4, according to the sequence reported previously (Gene Bank accession no. Y08915). Both cDNAs were subcloned into the BamHI site of pGEX3X vector. The constructs with truncated hα4 cDNAs were prepared as follows. Hα4 (1-93), (94-293), and (94-202) cDNA fragments were prepared by digestion with BamHI-HincII,HincII-EcoRI, and HincII-EcoRV of human α4 cDNA and were subcloned into BamHI/Sma I,Sma I/EcoRI, and Sma I sites of pGEX3X vector, respectively. Hα4 (1-293) and (210-293) were prepared from theEcoRI fragments of hα4 cDNAs isolated independently from RPMI8866 and IM9 cDNA libraries, respectively, and were subcloned into the EcoRI site of pGEX2T vector. The orientations and the reading frames of the cDNA inserts were verified by nucleotide sequencing of the final constructs. GST fusion proteins were prepared by affinity chromatography, as described.20
Cell lysis, immunoprecipitation, and Western blotting.
Cells were lysed in lysis buffer containing 1% Nonidet P-40, 150 mmol/L NaCl, 10 mmol/L Tris-Cl (pH 7.8), 1 mmol/L EDTA, 0.05% NaN3, 100 mmol/L NaVO4, 1 mmol/L phenylmethylsulfonylfluoride (PMSF), and 10 μg/mL aprotinin. The lysates were centrifuged for 5 minutes at 12,000g at 4°C to remove nuclei and insoluble materials and were used for immunoprecipitation as previously described19 or for the in vitro phosphatase assay. The lysates of 1 × 107 cells were incubated with specific Abs for 2 hours at 4°C. Immune complexes were collected with 30 μL of protein A-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden), washed 4 times with the lysis buffer, and then resuspended with sodium dodecyl sulfate (SDS) sample buffer. For the pull-down assay, the lysate of 5 × 107 cells was incubated with 10 μg of each fusion protein and the precipitated molecules were collected with Glutathione-Sepharose beads (Pharmacia). After SDS-polyacrylamide gel electrophoresis (SDS-PAGE), separated proteins were transferred onto nitrocellulose filters by electroblotting. PP2Ac or α4 was detected by anti-PP2Ac Ab or anti-hα4 Ab at the dilution of 1/1,000 or 1/400, respectively. The blots were developed using an enhanced chemiluminescence kit (Amersham Life Science, Tokyo, Japan) according to the manufacturer's protocol.
Proliferation assay.
Cells were cultured at 1 × 104 cells/well in 96-well microtiter plates containing 200 μL of RPMI-1640 culture medium with various concentrations of rapamycin for 48 hours. Relative cell numbers were analyzed by WST-1 assay.32 Cells were pulsed with 50 μL of WST-1 solution (1 mmol/L WST-1 and 20 mmol/L of 1-methoxy PMS), a compound of soluble tetrazolium (Dojindo, Kumamoto, Japan), for the last 4 hours. The absorbance was then measured with an enzyme-linked immunosorbent assay (ELISA) plate reader at a wavelength of 405 nm.
Transfection.
Hα4 cDNA was subcloned into pCDM8 expression vector. Cells were transfected with 20 μg of α4 cDNA and 2 μg of pSV2neo, which were linearized by Sac II and BamHI, respectively. These DNAs were transfected into Jurkat cells by the electroporation method, as previously described.33 After 2 days in the culture medium, transfected cells were selected in the presence of 1.0 mg/mL G418 (GIBCO, Grand Island, NY).
Phosphatase assay.
The holoenzyme of PP2A from rat brain was purified according to the protocol described previously,34 and the preparation of PP2A did not contain any activities of protein phosphatase 1, calcineurin, and protein phosphatase 2C. MBP or histone H1 was phosphorylated by cyclic AMP-kinase with 0.2 mmol/L [γ-32P]-ATP (3,000 to 5,000 cpm/pmol) for 60 minutes under standard assay conditions. Phosphorylated MBP or histone H1 were heat-treated at 65°C for 15 minutes to remove cyclic AMP kinase activity and collected by ammonium sulfate fractionation (0% to 80%) in the presence of 1 mg/mL bovine serum albumin (BSA). The proteins were washed 3 times with 80% ammonium sulfate and dialyzed against a buffer containing 10 mmol/L Tris-HCl (pH 7.5), 10 mmol/L 2-mercaptoethanol, and 10% (vol/vol) glycerol overnight. The standard assay system for dephosphorylation of MBP or histone H1 contained, in a final volume of 25 μL, 50 mmol/L imidazole-HCl (pH 7.0), 0.1% 2-mercaptoethanol (vol/vol), 1 mmol/L EDTA, 52 μg/mL of phosphorylated MBP or histone H1, and each sample of protein phosphatase 2A. The okadaic acid (OA)-sensitive phosphatase activity was calculated after measuring the phosphatase activities in vitro in the presence and absence of 50 nmol/L of okadaic acid (Sigma Chemicals Co, St Louis, MO), respectively. After 10 minutes of incubation at 30°C, 100 μL of 15% trichloroacetic acid was added, and the protein phosphatase activities were measured by the release of free32P from 32P-labeled substrates. All assays were performed in triplicate.
RESULTS AND DISCUSSION
Direct association of Igbp1 (α4) with PP2Ac.
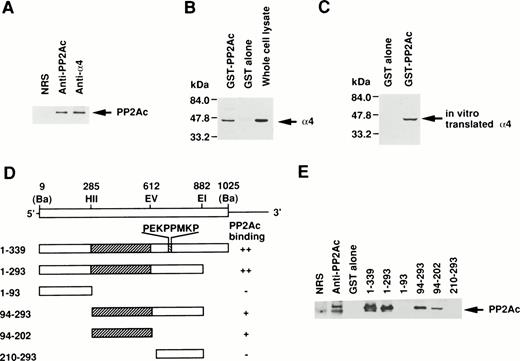
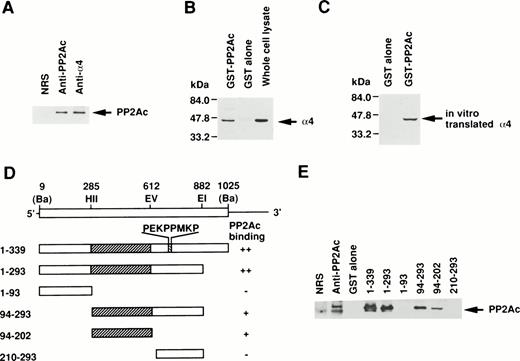
Because the direct association of α4 with PP2Ac was expected, we examined the coprecipitation of α4 and PP2A in lymphoid cells by anti-PP2Ac Western blot analysis. Cell lysate of Jurkat T-cells was first immunoprecipitated with the anti-hα4 Ab. Anti-PP2Ac Ab clearly detected a 39-kD PP2Ac in the α4 immunoprecipitate with rabbit anti-hα4 Ab as well as in whole cell lysates, indicating that PP2Ac was coprecipitated with α4 in Jurkat (Fig1A). To confirm the association of PP2Ac/α4, it was necessary to detect an association of α4 in the anti-PP2Ac immunoprecipitate. This reciprocal experiment failed due to the close migration of α4 to nonspecific bands from rabbit anti-hα4 Ab (data not shown). Therefore, we used a pull-down assay using a recombinant protein of GST-PP2Ac to precipitate the associated molecules from Jurkat. Cell lysates were mixed with affinity-purified GST-PP2Ac and precipitated with Glutathione-Sepharose beads. Western blot analysis of the precipitate with anti-hα4 Ab clearly detected a 45-kD band identical to the α4 recognized directly with the anti-hα4 Ab (Fig 1B). It was detected only with the GST-PP2Ac but not with the control GST. We confirmed similar results using WEHI 231 B cells (data not shown). These results demonstrate that α4 is associated with PP2Ac in lymphoid cells. PP2Ac associates with a regulatory subunit PR65, and this core dimer can further interact with various cellular regulatory components.23 24 To understand the molecular interaction directly, we studied the association of α4 and PP2Ac using a recombinant α4. Radiolabeled α4 synthesized in vitro by reticulocyte lysate from murine α4 cDNA in pGEM3Z vector by T7 polymerase in the presence of 35S-methionine was mixed with GST-PP2Ac. A 45-kD α4 protein was coprecipitated specifically with GST-PP2Ac but not with GST alone (Fig 1C), indicating that the association of α4 and PP2Ac did not require other cellular components from lymphocytes. Although the α4 protein contained the reticulocyte lysate, it suggested that the association of α4 protein with PP2Ac does not require conventional regulatory components for PP2Ac existing in lymphoid cells. In the same binding assay, we examined the involvement of rapamycin. Rapamycin did not directly induce the dissociation of α4 and PP2Ac (data not shown).
Association of α4 with PP2Ac. (A) Cell lysates of Jurkat were immunoprecipitated for 2 hours at 4°C with either anti-hα4 Ab, anti-PP2Ac Ab, or preimmune serum. The immunoprecipitates were separated by SDS/10% PAGE and transferred to a nitrocellulose filter. The filter was then probed with anti-PP2Ac Ab. The migration of human PP2Ac is indicated. (B) Cell lysates of Jurkat (50 × 106 cells) were mixed with 10 μg of GST-PP2Ac fusion protein or the control GST alone. The precipitates (15 × 106 cell equivalents per lane) were captured by Glutathione-Sepharose beads, separated by SDS/10% PAGE, and transferred to nitrocellulose filter. The filter was immunodetected with anti-hα4 Ab. Jurkat cell lysate (1 × 106/lane) was used as a positive control for α4 immunoblotting. The migration of α4 is indicated. (C) mα4 was synthesized in vitro in the presence of 35S-methionine using an in vitro translation kit (Amersham). GST-PP2Ac or GST alone was mixed with radiolabeled α4 and precipitated by Glutathione-Sepharose. Recovered proteins were separated by SDS-PAGE and subsequently developed by autoradiography. (D) Schematic diagram of GST-α4 fusion proteins used to localize the region that is necessary for binding to PP2Ac. Numbers on the left side indicate the positions of amino acid residues of α4. Restriction enzyme sites used for construction of mutants are shown. Ba,BamHI; EI, EcoRI; EV, EcoRV; HII,HincII. Hatched regions indicate the binding site. The intensities of the PP2Ac bands in the pull-down assay performed in (E) are indicated by ++, +, and −. (E) Various mutants of GST-α4 fusion proteins were tested for their binding activities to PP2Ac.
Association of α4 with PP2Ac. (A) Cell lysates of Jurkat were immunoprecipitated for 2 hours at 4°C with either anti-hα4 Ab, anti-PP2Ac Ab, or preimmune serum. The immunoprecipitates were separated by SDS/10% PAGE and transferred to a nitrocellulose filter. The filter was then probed with anti-PP2Ac Ab. The migration of human PP2Ac is indicated. (B) Cell lysates of Jurkat (50 × 106 cells) were mixed with 10 μg of GST-PP2Ac fusion protein or the control GST alone. The precipitates (15 × 106 cell equivalents per lane) were captured by Glutathione-Sepharose beads, separated by SDS/10% PAGE, and transferred to nitrocellulose filter. The filter was immunodetected with anti-hα4 Ab. Jurkat cell lysate (1 × 106/lane) was used as a positive control for α4 immunoblotting. The migration of α4 is indicated. (C) mα4 was synthesized in vitro in the presence of 35S-methionine using an in vitro translation kit (Amersham). GST-PP2Ac or GST alone was mixed with radiolabeled α4 and precipitated by Glutathione-Sepharose. Recovered proteins were separated by SDS-PAGE and subsequently developed by autoradiography. (D) Schematic diagram of GST-α4 fusion proteins used to localize the region that is necessary for binding to PP2Ac. Numbers on the left side indicate the positions of amino acid residues of α4. Restriction enzyme sites used for construction of mutants are shown. Ba,BamHI; EI, EcoRI; EV, EcoRV; HII,HincII. Hatched regions indicate the binding site. The intensities of the PP2Ac bands in the pull-down assay performed in (E) are indicated by ++, +, and −. (E) Various mutants of GST-α4 fusion proteins were tested for their binding activities to PP2Ac.
Next, we studied the specific binding site of α4 to PP2Ac. Deletion mutants of α4 prepared as GST-fusion proteins were used to determine the binding region for PP2Ac. Western blot analysis with anti-PP2Ac Ab showed that the middle region of α4 encompassing 109 amino acids was necessary and sufficient for the binding to PP2Ac but that the amino- or carboxyl-terminal portion did not bind to PP2Ac in this assay (Fig1D and E). The amino acid sequence, required for binding to PP2Ac, did not show any unique motif. The SH3-binding consensus motif (PEKPPMKP) present in α420 was not involved in this interaction. A similar association of PP2A was recently shown for Hox11 and eRF1. Hox11 is capable of binding to PP2Ac directly, inhibiting cell cycle arrest at the transition from G2 to M phase. The interaction site was narrowed down to amino acids 149 to 199 of Hox11.29 The carboxyl-terminal side 43 amino acids of human eRF1 (amino acids 338-381) are responsible for the binding to PP2Ac.28 In a homology comparison of the amino acid sequences, no obvious similarity was found to the binding site on α4 (data not shown).
Involvement of PP2Ac/α4 in rapamycin-sensitive signal transduction pathway.
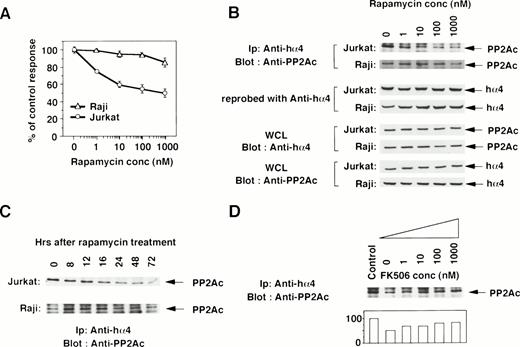
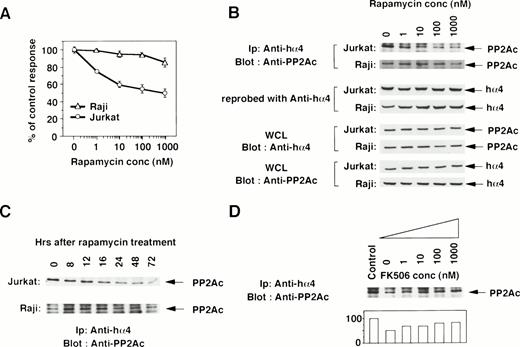
To determine the functional contribution of the PP2Ac/α4 complex in lymphocytes, we tested whether rapamycin affects the association of α4 with PP2Ac. Rapamycin sensitivity varies among cell lines.35 The growth of a T-cell line Jurkat was sensitive to rapamycin treatment, but that of a B-cell line Raji was resistant (Fig 2A). Jurkat cells were treated with various concentrations of rapamycin, and the association was monitored by immunoprecipitation with anti-hα4 Ab followed by immunoblot with anti-PP2Ac Ab. Rapamycin treatment induced the dissociation of PP2Ac from α4 in a dose-dependent manner (Fig 2B). The concentration of rapamycin that induced the dissociation of PP2Ac/α4 was comparable to that required for growth inhibition. Dissociation of PP2Ac from α4 was discernible after 12 hours and almost no PP2Ac remained in association with α4 after 48 to 72 hours of treatment (Fig 2C). The amount of α4 protein was quite similar in each lane, as confirmed on the same filter by reprobing with anti-α4 antibody (Fig 2B). Existence of PP2Ac and hα4 proteins was also confirmed by Western blot with the whole cell lysates (Fig 2B). Interestingly, rapamycin could not dissociate α4 from PP2Ac in rapamycin-resistant Raji cells (Fig 2B and C). Furthermore, we examined whether the dissociation of the α4/PP2Ac by the treatment of rapamycin was inhibited in the presence of FK506, which binds to the same binding molecule FKBP (Fig2D). Increasing concentrations of FK506 recovered the association of α4 and PP2Ac. PP2A is obviously associated with a number of signaling molecules in a variety of cell types,23 24 but none of the molecules was shown to be affected by rapamycin treatment. This result is, to our knowledge, the first report in which rapamycin treatment dissociates the complex structure composed of PP2Ac.
Rapamycin-sensitive dissociation of PP2Ac and α4. (A) Rapamycin sensitivity was measured on the proliferation of Jurkat and Raji. Jurkat is sensitive to rapamycin treatment, but Raji is relatively insensitive. Relative cell proliferation was compared after measuring by WST-1 assay.31 (B) Rapamycin induces the dose-dependent dissociation of PP2Ac/α4 complex in rapamycin-sensitive Jurkat but not in rapamycin-resistant Raji. Cell lysates were prepared after the culture and the PP2Ac coprecipitated with α4 is detected by Western blot analysis. After detecting signals, the probe on the filter was stripped off according to the company's protocol (Amersham). The filter was then reprobed with anti-hα4 Ab to see the amount of hα4 protein in each lane. To further confirm the existence of similar amounts of PP2Ac and hα4 proteins, Western blot analysis was performed with the whole cell lysates (WCL). (C) Both Jurkat and Raji cells were treated with rapamycin (330 nmol/L) and the PP2Ac associated with α4 was detected by Western blot analysis. Cells were harvested at the time points indicated and the immunoblot was developed with anti-PP2Ac Ab. The migration of PP2Ac is as indicated. (D) Effect of FK506 was examined during the treatment of rapamycin. Varying concentrations of FK506 were added in the culture of Jurkat with rapamycin (10 nmol/L). The cells were harvested after 48 hours and lysed as described above. The α4-associated PP2Ac was detected on Western blot analysis. The PP2Ac signals were measured by the densitometric analysis and are shown as the relative intensities (%) of control culture in the absence of rapamycin. The amounts of hα4 immunoprecipitated with anti-hα4 Ab were controlled by reprobing the same filter (data not shown).
Rapamycin-sensitive dissociation of PP2Ac and α4. (A) Rapamycin sensitivity was measured on the proliferation of Jurkat and Raji. Jurkat is sensitive to rapamycin treatment, but Raji is relatively insensitive. Relative cell proliferation was compared after measuring by WST-1 assay.31 (B) Rapamycin induces the dose-dependent dissociation of PP2Ac/α4 complex in rapamycin-sensitive Jurkat but not in rapamycin-resistant Raji. Cell lysates were prepared after the culture and the PP2Ac coprecipitated with α4 is detected by Western blot analysis. After detecting signals, the probe on the filter was stripped off according to the company's protocol (Amersham). The filter was then reprobed with anti-hα4 Ab to see the amount of hα4 protein in each lane. To further confirm the existence of similar amounts of PP2Ac and hα4 proteins, Western blot analysis was performed with the whole cell lysates (WCL). (C) Both Jurkat and Raji cells were treated with rapamycin (330 nmol/L) and the PP2Ac associated with α4 was detected by Western blot analysis. Cells were harvested at the time points indicated and the immunoblot was developed with anti-PP2Ac Ab. The migration of PP2Ac is as indicated. (D) Effect of FK506 was examined during the treatment of rapamycin. Varying concentrations of FK506 were added in the culture of Jurkat with rapamycin (10 nmol/L). The cells were harvested after 48 hours and lysed as described above. The α4-associated PP2Ac was detected on Western blot analysis. The PP2Ac signals were measured by the densitometric analysis and are shown as the relative intensities (%) of control culture in the absence of rapamycin. The amounts of hα4 immunoprecipitated with anti-hα4 Ab were controlled by reprobing the same filter (data not shown).
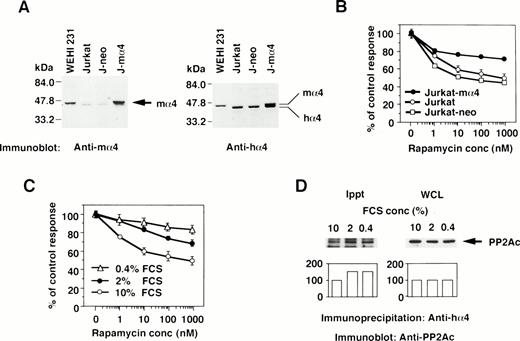
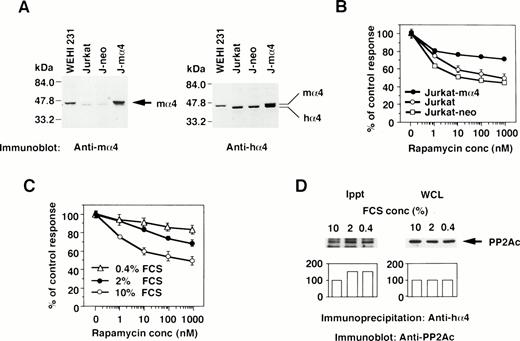
The effect of the increased α4 expression was examined by DNA transfection of mα4 into human cells. The introduced mα4 was clearly identified as a larger band on Western blot analysis by the anti-mα4 Ab. Jurkat-mα4 transfectant expressed mα4 in addition to hα4 detected by the anti-hα4 Ab, but parental Jurkat cells and Jurkat transfectants with neomycin-resistant gene alone (Jurkat-neo) expressed hα4 only (Fig 3A). Rapamycin inhibited the proliferation of Jurkat cells, as shown in Fig 3B. Jurkat transfected with mα4, expressing the double amounts of α4 protein, became less susceptible to rapamycin in comparison to parental Jurkat cells (Fig 3B). The control transfectant expressing neomycin alone was as sensitive to rapamycin as parental Jurkat cells. Mα4 is 93% homologous to hα4 at amino acid level (data not shown), and we assumed that this highly conserved structure of mα4 allowed it to function additively with hα4 in Jurkat cells. Because conditions of cell culture affect the rapamycin sensitivity,36 we tested whether a reduced concentration of serum in culture medium influences the expression of α4 and its association with PP2Ac. Jurkat cells cultured in medium containing less than 2% fetal calf serum (FCS) became more resistant to rapamycin than those cultured with 10% FCS (Fig 3C). Low concentration of serum did not change the expression level of α4 (data not shown) and PP2A (Fig 3D; WCL) themselves; however, the association of α4 and PP2A was augmented in Jurkat when cultured in the medium with less than 2% FCS (Fig 3D; Ippt). These results indicate that the PP2A/α4 association functions as a new target of rapamycin in lymphoid cells.
The correlation of α4 expression and rapamycin sensitivity. (A) Expression of α4 in transfectants was shown by Western blot analysis. Jurkat transfectants expressing mα4 or neomycin-resistant gene, parental Jurkat, and mouse B-cell line WEHI 231 were lysed in lysis buffer containing 1% NP-40. The immunoblot was developed with anti-mα4 Ab (left side) or anti-hα4 Ab (right side). Anti-hα4 Ab recognizes both hα4 and mα4 proteins. The migrations of mouse and hα4 are as indicated. (B) Cells were cultured at 1 × 104/well in 200 μL of medium with various concentrations of rapamycin for 48 hours. Relative cell numbers were analyzed by WTS-1 assay. Results are shown as the percentage of the control culture without rapamycin. Data are representative of 4 independent experiments and are shown as the mean of duplicate samples ± standard deviations. (C) Jurkat cells were cultured at 1 × 105/mL with various concentrations of serum as 10%, 2%, or 0.4% for 72 hours. Rapamycin sensitivity was measured by the WTS-1 assay as described above. (D) Jurkat cells were cultured in the medium that contained either 10%, 2%, or 0.4% of FCS for 72 hours. The amounts of PP2Ac bound to α4 were detected after immunoprecipitation with anti-hα4 Ab, followed by anti-PP2Ac Western blot analysis. Total amounts of PP2Ac were detected using whole cell lysate (WCL). Columns below the bands show the arbitrary units to indicate the relative intensity of the bands as determined by a densitometer.
The correlation of α4 expression and rapamycin sensitivity. (A) Expression of α4 in transfectants was shown by Western blot analysis. Jurkat transfectants expressing mα4 or neomycin-resistant gene, parental Jurkat, and mouse B-cell line WEHI 231 were lysed in lysis buffer containing 1% NP-40. The immunoblot was developed with anti-mα4 Ab (left side) or anti-hα4 Ab (right side). Anti-hα4 Ab recognizes both hα4 and mα4 proteins. The migrations of mouse and hα4 are as indicated. (B) Cells were cultured at 1 × 104/well in 200 μL of medium with various concentrations of rapamycin for 48 hours. Relative cell numbers were analyzed by WTS-1 assay. Results are shown as the percentage of the control culture without rapamycin. Data are representative of 4 independent experiments and are shown as the mean of duplicate samples ± standard deviations. (C) Jurkat cells were cultured at 1 × 105/mL with various concentrations of serum as 10%, 2%, or 0.4% for 72 hours. Rapamycin sensitivity was measured by the WTS-1 assay as described above. (D) Jurkat cells were cultured in the medium that contained either 10%, 2%, or 0.4% of FCS for 72 hours. The amounts of PP2Ac bound to α4 were detected after immunoprecipitation with anti-hα4 Ab, followed by anti-PP2Ac Western blot analysis. Total amounts of PP2Ac were detected using whole cell lysate (WCL). Columns below the bands show the arbitrary units to indicate the relative intensity of the bands as determined by a densitometer.
Regulation of PP2A activity by α4 molecule.
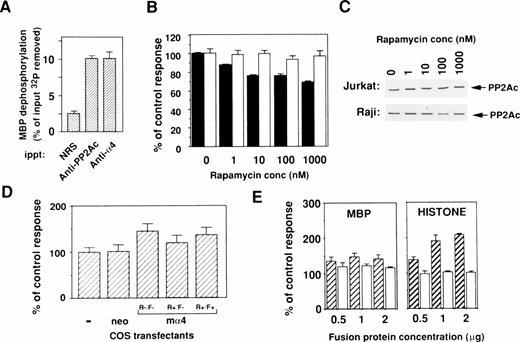
To evaluate the catalytic activity of PP2Ac bound to α4, an in vitro phosphatase assay was performed using OA, the specific inhibitor for PP2A activity. All results are shown as the OA-sensitive phosphatase activity. PP2Ac coimmunoprecipitated with α4 showed phosphatase activity upon [32P]-radiolabeled substrate MBP in a similar way as PP2Ac immunoprecipitated with anti-PP2Ac antibody (Fig 4A). This result clearly indicates that PP2Ac is associated with α4 in lymphocytes and suggests that rapamycin treatment may alter the activity of PP2A. Because many PP2Ac-associated molecules have negative regulatory functions on phosphatase activity,23 24 we tested whether phosphatase activity might change in the cells by the dissociation of PP2Ac/α4 complex. Therefore, the amount of PP2Ac on Western blot analysis and the phosphatase activities in total cell lysates were examined after rapamycin treatment. Interestingly, rapamycin treatment induced a downregulation of phosphatase activity in rapamycin-sensitive Jurkat, but the activity did not change in rapamycin-resistant Raji (Fig 4B). The decrease of phosphatase activity in Jurkat cells after rapamycin treatment is not as marked as the dissociation state of the α4/PP2Ac complex. Repeated experiments showed similar results (data not shown), suggesting that the PP2Ac activity might be regulated by various regulatory molecules in lymphoid cells. Rapamycin treatment of various concentrations did not alter the level of PP2Ac expression in both Jurkat and Raji (Fig 4C). These results suggest that association with α4 maintains higher phosphatase activity of PP2Ac in lymphoid cells. To further study a regulatory function of α4 on PP2A activity, enzymatic activity was compared before and after α4 cDNA transfection into COS-7 cells. The α4-transfected COS-7 showed the increased PP2A activity when compared with mock-transfected COS-7 cells (Fig 4D). The change of phosphatase activity was also affected in the presence of rapamycin in the α4-COS transfectant, which was again recovered by the addition of FK506 (Fig 4D). These results further support the idea that α4 is involved as a target of rapamycin and is functionally composed in the rapamycin/FKBP complex. The rapamycin resistance of lymphocytes is probably controlled by the association with PP2Ac.
Binding of phosphatase activity to α4 in cells. (A) Cell lysates of Jurkat were immunoprecipitated by either anti-hα4 Ab, anti-PP2Ac Ab, or normal rabbit serum (NRS). Phosphatase activities in the complex were assayed using 32P-labeled MBP as a substrate. Results are shown as the percentage of input 32P dephosphorylated from MBP. Optimal enzyme and substrate ratio and the incubation time were determined with the rat PP2A purified as described previously.33 Data are the mean of duplicate samples + standard deviations. (B) Jurkat (▪) or Raji (□) cells were treated with various concentrations of rapamycin for 48 hours. The cells were lysed and assayed for their phosphatase activity using 32P-labeled MBP as a substrate. The activity was measured in the presence or absence of 50 nmol/L of OA and the value without OA subtracted from that with OA was calculated. For comparison, the value of lysate without rapamycin was set as 100%. Protein concentrations of samples were adjusted and the amounts of PP2Ac protein were shown in (C). (C) Western blot analysis to demonstrate the amounts of PP2Ac in all samples. Results indicate that proteins are equally adjusted before measuring the phosphatase activities. (D) COS-7 cells were transfected with the mα4 cDNA in pCDM8 or control vector. Cells were lysed after 72 hours of culture and the phosphatase assay was performed as in (B). The effect of rapamycin (10 nmol/L) was measured on mα4-COS transfectant (R+/F−) in comparison to the culture without rapamycin (R−/F−). Recovery of phosphatase activity was measured by the addition of FK506 (100 nmol/L) (R+/F+). (E) Upregulation of phosphatase activity by α4. Effect of GST-α4 (▨) or GST alone (□) was measured in the in vitro phosphatase assay using the phosphorylated MBP or histone H1 with purified PP2A. Regulatory activity on the PP2A was shown as the percentage of control measured only with substrate and enzyme.
Binding of phosphatase activity to α4 in cells. (A) Cell lysates of Jurkat were immunoprecipitated by either anti-hα4 Ab, anti-PP2Ac Ab, or normal rabbit serum (NRS). Phosphatase activities in the complex were assayed using 32P-labeled MBP as a substrate. Results are shown as the percentage of input 32P dephosphorylated from MBP. Optimal enzyme and substrate ratio and the incubation time were determined with the rat PP2A purified as described previously.33 Data are the mean of duplicate samples + standard deviations. (B) Jurkat (▪) or Raji (□) cells were treated with various concentrations of rapamycin for 48 hours. The cells were lysed and assayed for their phosphatase activity using 32P-labeled MBP as a substrate. The activity was measured in the presence or absence of 50 nmol/L of OA and the value without OA subtracted from that with OA was calculated. For comparison, the value of lysate without rapamycin was set as 100%. Protein concentrations of samples were adjusted and the amounts of PP2Ac protein were shown in (C). (C) Western blot analysis to demonstrate the amounts of PP2Ac in all samples. Results indicate that proteins are equally adjusted before measuring the phosphatase activities. (D) COS-7 cells were transfected with the mα4 cDNA in pCDM8 or control vector. Cells were lysed after 72 hours of culture and the phosphatase assay was performed as in (B). The effect of rapamycin (10 nmol/L) was measured on mα4-COS transfectant (R+/F−) in comparison to the culture without rapamycin (R−/F−). Recovery of phosphatase activity was measured by the addition of FK506 (100 nmol/L) (R+/F+). (E) Upregulation of phosphatase activity by α4. Effect of GST-α4 (▨) or GST alone (□) was measured in the in vitro phosphatase assay using the phosphorylated MBP or histone H1 with purified PP2A. Regulatory activity on the PP2A was shown as the percentage of control measured only with substrate and enzyme.
Next, we attempted to directly demonstrate a positive regulation of PP2Ac activity with α4 protein. Affinity-purified GST-α4 protein was mixed with purified PP2A in the in vitro phosphatase assay using phosphorylated MBP as a substrate. The phosphatase activity was augmented in the presence of α4 protein (Fig 4E). The effect was obvious on phosphorylated-histone (right panel), but less on phosphorylated-MBP (left panel) and -casein (data not shown) as substrates. The augmentation of phosphatase activity was not observed with other GST-fusion proteins such as with truncated α4 lacking the amino acids (94-202) (data not shown). Many PP2A-binding molecules either negatively regulate phosphatase activity23,24 or do not exhibit any modulating activity, such as eRF1.28Recently, Heriche et al30 reported that PP2A is directly associated with CK2α, whose catalytic activity appeared to enhance PP2A activity and is presumably involved in deactivation of the mitogen-activated protein kinase pathway.
We have demonstrated that α4 is involved in the rapamycin-sensitive signal transduction pathway through the association with PP2Ac and probably controls phosphorylation states of certain functional molecules involved in cell cycle progression. PP2A dephosphorylates and inactivates several of the growth factor-stimulated protein kinases in vitro, suggesting that PP2A normally functions as a suppressor of cell growth.23,24 Growth factor-stimulated protein kinases (MAPK/ERKs) phosphorylate a number of substrates in addition to 90-kD S6 kinase and several transcription factors.37 Treatment of active preparations of MAPK/ERK with the catalytic subunit of PP2A causes dephosphorylation of phospho-threonine and inhibition of kinase activity.38 Treatment of several different cell types with OA causes activation of MAPK/ERKs,39 demonstrating that a constitutive level of serine/threonine phosphatase activity is necessary to maintain MAPK/ERKs in a low-activity state. In our report, rapamycin inhibits the proliferation of cells by dissociating PP2Ac from α4. The results suggest that PP2A may function under certain conditions as a positive regulator of cell proliferation.
Rapamycin/FKBP complex interacts directly with mTOR and inhibits its enzymatic activity. Signals induced by growth factors activate mTOR, which then activates S6K40 and PHAS1.41 PHAS1 is a direct substrate of mTOR, but the interaction of S6K with mTOR is indirect and the mechanism of S6K activation by mTOR is not determined yet. The mechanism of inhibiting activity of p27 degradation by rapamycin is not clarified either.42,43 In our report, we demonstrated that rapamycin disrupts the PP2A/α4 association, which is now considered as a target of rapamycin in the growth inhibition of lymphoid cells. Di Como and Arndt21 suggested that Tap42/SIT4 or Tap42/PP2A might be located downstream of TOR signaling in yeast. It is important to identify the molecular interaction between PP2A/α4 and mTOR. The PP2A/α4 signaling pathway might be also involved in B-cell activation, because rapamycin potentially suppresses the B-cell activation by BCR44 or cytokine stimulation.45
During the preparation of this manuscript, Murata et al46reported that α4 binds to PP2Ac. Our results presented here as well as theirs suggest that the Igbp1 (α4) is involved in the rapamycin-sensitive signal transduction pathway, which might regulate the PP2Ac activity for the cell cycle progression of lymphocytes.
Supported by the grants from the Ministry of Education, Sports, Science and Culture, Japan.
Address reprint requests to Nobuo Sakaguchi, MD, Department of Immunology, Kumamoto University School of Medicine, 2-2-1, Honjo, Kumamoto 860, Japan; e-mail: nobusaka@kaiju.medic.kumamoto-u.ac.jp
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.