Abstract
von Willebrand factor (vWF) is a multimeric adhesive glycoprotein with one factor VIII binding site/subunit. Prior reports suggest that posttranslational modifications of vWF, including formation of N-terminal intersubunit disulfide bonds and subsequent cleavage of the propeptide, influence availability and/or affinity of factor VIII binding sites. We found that deletion of the vWF propeptide produced a dimeric vWF molecule lacking N-terminal intersubunit disulfide bonds. This molecule bound fluorescein-labeled factor VIII with sixfold lower affinity than multimeric vWF in an equilibrium flow cytometry assay (approximate KDs, 5 nmol/L v 0.9 nmol/L). Coexpression of propeptide-deleted vWF with the vWF propeptide in trans yielded multimeric vWF that displayed increased affinity for factor VIII. Insertion of an alanine residue at the N-terminus of the mature vWF subunit destroyed binding to factor VIII, indicating that the native mature N-terminus is required for factor VIII binding. The requirement for vWF propeptide cleavage was shown by (1) a point mutation of the vWF propeptide cleavage site yielding pro-vWF that was defective in factor VIII binding and (2) correlation between efficiency of intracellular propeptide cleavage and factor VIII binding. Furthermore, in a cell-free system, addition of the propeptide-cleaving enzyme PACE/furin enabled factor VIII binding in parallel with propeptide cleavage. Our results indicate that high-affinity factor VIII binding sites are located on N-terminal disulfide-linked vWF subunits from which the propeptide has been cleaved.
FACTOR VIII FUNCTIONS in the intrinsic pathway of blood coagulation as a cofactor to accelerate the activation of factor X by factor IXa, a reaction that occurs on phosphatidylserine (PS)-containing membranes in the presence of calcium ions.1Deficiency in factor VIII is the cause of the X-chromosome linked bleeding disorder hemophilia A.2 Factor VIII circulates in plasma bound to von Willebrand factor (vWF) in a noncovalent protein complex. While bound to vWF in plasma, factor VIII is prevented from binding to PS-containing membranes, an interaction that inhibits both binding to factor IXa and degradation by activated protein C.3,4 In addition, the presence of vWF in the conditioned medium can promote stable accumulation of factor VIII secreted from transfected cells in culture.5 6
vWF is a large adhesive glycoprotein that is synthesized in megakaryocytes and endothelial cells. The primary amino acid sequence of vWF identified homologous domains arranged in the order D1-D2-D′-D3-A1-A2-A3-D4.7-9 vWF is synthesized as a precursor with a large propeptide, encompassing domains D1-D2, that is cleaved upon transport through the secretory apparatus at the Arg-Ser bond at residue 763-764. Fully processed vWF is a multimeric protein consisting of disulfide-bonded monomeric subunits that range from dimers to multimers extending up to 20 × 106Daltons.10,11 Initial dimerization mediated through disulfide linkages within the carboxyl (C)-terminal region of the protein occurs within the endoplasmic reticulum.12-14The vWF propeptide is required to promote polymerization of vWF dimers in the trans-Golgi compartment through disulfide bonds between D3 domains of opposing subunits.15,16 Identified factor VIII binding site determinants lie within the D′ domain at the amino (N)-terminus of mature vWF subunits.17-20
In endothelial cells, multimeric vWF assembly proceeds to a high degree before propeptide cleavage.21 Furthermore, mutagenesis studies have shown that propeptide cleavage is not required for vWF multimerization.15,16 Constitutively secreted vWF from endothelial cells as well as vWF from recombinant expression systems is not completely processed, with some subunits retaining the propeptide.9,13,22 Coexpression of PACE/furin, an intracellular propeptide processing enzyme, with vWF enhances the secretion of completely processed vWF.23,24 Deletion of the vWF propeptide produces efficiently secreted dimeric vWF (ΔPro-vWF) that is not multimerized, indicating that the propeptide is necessary to promote the formation of N-terminal intersubunit disulfide bonds.15,16 However, coexpression of the propeptide with ΔPro-vWF leads to limited multimerization via N-terminal disulfide bond formation, indicating that the propeptide does not have to be covalently linked to vWF to promote multimerization.15
Although each subunit of a vWF multimer contains one factor VIII binding site, the ratio of circulating factor VIII to vWF observed in vivo is approximately 1:50.25-28 In vitro binding studies have yielded conflicting data for the number of available factor VIII binding sites/vWF subunit, with reported ratios from 1:1 to as low as 1:70.29-33 Although the source for the differences remains uncertain, these reports raise the possibility that either not all factor VIII binding sites are accessible or that affinity of sites is altered by other factors. Because each circulating vWF molecule contains 2 terminal non–disulfide-linked N-termini and 2-80 internal disulfide-linked N-termini, altered binding due to disulfide bond formation may influence the location of factor VIII on vWF molecules in vivo. Determining the relationship between the maturation events of disulfide bond formation and propeptide cleavage and formation of high affinity factor VIII binding sites is a chief motivation of the following studies.
The influence of vWF processing events on factor VIII binding was previously studied by expression of site-directed mutants of vWF and yielded conflicting results.34,35 The propeptide cleavage site mutation R763K at the P1 residue yielded pro-vWF that did not bind or stabilize factor VIII expressed from Chinese hamster ovary (CHO) cells.34 Deletion of the propeptide yielded dimeric vWF that was capable of binding and stabilizing factor VIII expressed from CHO cells.34 Although these studies were not designed to quantitate binding affinity, they suggested that propeptide removal was required to expose a factor VIII binding site. In contrast, studies using a different expression system and a different method of analysis with a similar propeptide deletion mutant that contained an extra alanine at the junction of the signal peptide and the mature vWF sequences (vWFdelpro),35 designated ΔPro+A herein, did not detectably bind factor VIII. To elucidate the structural requirements for vWF binding to factor VIII, we have examined the binding of factor VIII to vWF containing and lacking the propeptide, vWF containing and lacking N-terminal intersubunit disulfide bonds, and dimeric vWF containing and lacking the N-terminal alanine residue. Our studies demonstrate that formation of N-terminal disulfide bonds between vWF dimers is necessary for formation of the highest affinity factor VIII binding sites and that these sites remain inaccessible until the propeptide has been cleaved.
MATERIALS AND METHODS
Materials
Fetal bovine serum (FBS) and Dulbecco's Modified Essential Medium (DMEM) were obtained from Mediatech (Herndon, VA). OptiMEM medium and goat antirabbit secondary antibody were purchased from GIBCO-BRL (Gaithersburg, MD). Aprotinin and phenylmethyl sulfonyl fluoride (PMSF) were from Boehringer Mannheim (Chicago, IL). Bovine brain PS, phosphatidyl ethanolamine synthesized by transphosphatidylation of egg phosphatidyl-choline, and egg phosphatidyl-choline were from Avanti Polar Lipids Inc (Alabaster, AL). Cholesterol was from Calbiochem (San Diego, CA). Soybean trypsin inhibitor, bovine serum albumin (BSA), and o-phenylenediamine dihydrochloride (OPD) tablets were from Sigma Corp (St Louis, MO). Unless otherwise noted, anti-vWF polyclonal antibody and anti-vWF horseradish peroxidase (HRP)-conjugated antibody were from Dako Corp (Carpinteria, CA). Fluorescein-5-maleimide and fluorescein isothiocyanate (FITC) were purchased from Molecular Probes, Inc (Eugene, OR). Superose-12 beads (34 μm) were purchased from Pharmacia Biotech (Piscataway, NJ). Centricon and Microcon concentrators were purchased from Amicon Corp (Danvers, MA). Immulon 2 membranes and microtiter plates were from Dynatech (Chantilly, VA). vWF normal reference plasma was from Bio/Data Corp (Horsham, PA). The enhanced chemiluminescence system (ECL) was from Amersham Corp (Arlington Heights, IL). Cryoprecipitate was obtained from the American Red Cross. Recombinant factor VIII, monoclonal antibody (MoAb) to factor VIII, F8,36 and anti-vWF MoAbs vW/13.7.9 and vW/16.9.2 were generously provided by D. Pittman (Genetics Institute Inc, Cambridge, MA).
Fluorescein Labeling
Factor VIII was labeled with fluorescein-5-maleimide, as described previously.3,37 Protein concentration of factor VIII was determined using a micro-bicinchoninic acid assay (Pierce, Rockford, IL) using BSA as a standard. Anti-vWF MoAb vW/16.9.2 was labeled with FITC as described.38 Protein concentration was measured by absorbance at 280 nm with correction for fluorescein absorbance at 280 nm (A280 − 0.21 × A490). Efficiency of labeling was determined by comparing absorbance at 490 nm to corrected absorbance at 280 nm.
Expression Vectors
pMT2-vWF,15 pMT2-KRX (the vWF propeptide alone), pMT2-ΔPro-vWF, and ΔPro+A were previously described.15,35 The expression vectors encoding wild-type PACE/furin (pMT2-PACE) and mutant PACE/furin (pMT2-PACE Sol and pMT2-PACE SA) and vWF propeptide cleavage site mutant molecules (pMT2vWF-R760K and pMT2-vWF-RXK/KXD, herein described as R760K/K762D) were previously described.9,15,23 39Escherichia coli DH5α was used to prepare plasmid DNA that was purified by standard cesium chloride-ethidium bromide gradient equilibrium centrifugation.
vWF Expression in COS-1 and CHO Cells
COS-1 monkey cells were transfected with plasmid DNA by the diethylaminoethyl dextran procedure.40 Cells were maintained in 10% FBS in DMEM. At 48 hours posttransfection, transfected cells were washed with phosphate-buffered saline (PBS) to remove residual serum and incubated in OptiMEM medium containing 0.2 mg/mL aprotinin. After 24 hours, conditioned medium was harvested and fresh protease inhibitors were added (0.2 mg/mL aprotinin, 1 mg/mL soybean trypsin inhibitor, and 1 mmol/L PMSF). The concentration of vWF in the conditioned medium was measured by enzyme-linked immunosorbent assay (ELISA). The stably transfected CHO cell lines vWF-wt and vWF-ΔPro were previously described.34
vWF Preparation and Evaluation
Human plasma-derived vWF was purified from cryoprecipitate on sepharose CL4-b (Pharmacia Biotech) equilibrated with 20 mmol/L imidazole, 20 mmol/L ε-n-caproic acid, 150 mmol/L sodium chloride, 10 mmol/L sodium citrate, and 0.02% sodium azide, pH 6.5. Its concentration was measured by ELISA, using the polyclonal anti-vWF (and HRP-conjugated anti-vWF) antibodies cited above. vWF multimers were analyzed by gel electrophoresis on discontinuous sodium dodecyl sulfate (SDS)-agarose gels based on methods described previously.11 Where indicated, vWF was purified from conditioned medium of transfected cells by immunoaffinity chromatography as described.34
Preparation of MoAb vW/13.7.9-Coated Superose-12 Microspheres
Superose-12 microspheres (500 μL) were first equilibrated with 1 mol/L CO3Na2/HNaCO3 buffer, pH 11. CNBr (500 μg) dissolved in 500 μL acetonitrile was added to the microspheres and incubated for 10 minutes on an ice bath while swirling. If necessary, 4 N NaOH was added to the mix to maintain the pH between 10.5 and 11. The microspheres were extensively washed with distilled H2O, 95% acetone, and 200 mmol/L Na2CO3/HNaCO3 buffer, pH 9.75. Anti-vWF MoAb vW/13.7.9 (500 μL, 0.75 mg/mL) was added to the microspheres slur and incubated overnight at room temperature on a rocker. The beads were washed twice with 0.2 mol/L Na2CO3/HNaCO3, pH 9.75, and once with 1 mol/L NaCl, 0.05 mol/L Na2CO3/HNaCO3. Nonspecific active sites were blocked by incubating the coated beads with 10 vol of 100 mmol/L Tris-HCl 4 hours to overnight at room temperature with gentle mixing. Beads were kept at 4°C until used. The microsphere concentration was counted using a ZM Coulter-counter (Coulter Instruments, Hialeah, FL).
Factor VIII-vWF Binding Assays
VIII-vWF Binding Assays on Microtiter Plates
Factor VIII-vWF binding assays on microtiter plates were conducted using two methods. First, Immulon 2 microtiter plates were coated with 5 μg/mL anti-vWF polyclonal antibody in 50 mmol/L Na2CO3/HNaCO3, pH 9.6. Plates were subsequently washed after each step with TBST buffer (50 mmol/L Tris HCl, pH 7.6, 150 mmol/L NaCl, and 0.05% Tween 20). Plates were blocked for 1 hour at 37°C in TBST buffer with 3% BSA. Increasing amounts of recombinant factor VIII were preincubated with COS-1 cell conditioned medium containing vWF (100 ng/mL of conditioned medium), and this was added to the plates. After incubation, the amount of factor VIII bound to immobilized vWF was measured by factor VIII Coatest endpoint assay (Kabi Pharmacia, Uppsala, Sweden). The optical densities at 490 nm were read using an EL340 microtiter plate Bio Kinetics Reader (Bio-Tek Instruments Inc, Winooski, VT). In the second method, the buffers and washes were the same as in the first method. Microtiter plates were coated with anti-factor VIII MoAb F8 at 2 μg/mL in carbonate buffer. Conditioned medium from transfected COS-1 cells was incubated with increasing concentrations of recombinant factor VIII in TBST buffer containing 10 mmol/L CaCl2 and 3% BSA in polypropylene tubes at 37°C for 2 hours. After incubation, the conditioned medium/factor VIII samples were added to coated and blocked microtiter plates for 2 hours at 37°C. The amount of vWF bound to immobilized factor VIII was measured by adding anti-vWF HRP-conjugated antibody to the plate at 10 μg/mL. After the addition of OPD, the reaction was stopped by the addition of 2.5 mol/L H2SO4. The optical density was read and analyzed as described above.
Flow Cytometry/Equilibrium Binding Assays
Factor VIII binding to antibody immobilized vWF.
vWF (117 ng) was added to 200,000 MoAb vW/13.7.9-Superose microspheres in a final volume of 50 μL with TBS containing 0.01% Tween 80 and 0.1% BSA, pH 7.85 (TBST-BSA), and incubated at room temperature while shaking in a vortex mixer. After 45 minutes, the microsphere suspension was diluted with 50 μL TBST-BSA. At time zero, aliquots containing 6,500 vWF-microspheres were incubated with increasing concentrations of fluorescein-labeled factor VIII (in buffer containing 0.5 mmol/L CaCl2) or FITC-MoAb vW/16.9.2 in a final volume of 100 μL for 15 minutes. The samples were diluted to 500 μL before reading the fluorescence/microsphere by flow cytometry within 30 seconds of dilution. Equilibrium binding data were analyzed using the equation
where F is the measured fluorescence/Superose bead, F0 is the fluorescence for Superose bead in the absence of fluorescein-labeled factor VIII, Fmax is the maximum fluorescence with saturating concentrations of factor VIII, KD is the dissociation constant for factor VIII binding to vWF, and VIIIf is the molar concentration of fluorescein-labeled factor VIII. The term kf is the measured fluorescence per concentration unit of free factor VIII due to unbound factor VIII and any nonspecific binding of fluorescein-labeled factor VIII to control Superose beads lacking vWF. We assumed that the fraction of total factor VIII bound could be neglected (the nominal concentration of factor VIII binding sites was 0.1 nmol/L, assuming 1 binding site/vWF subunit). The variables, KD and Fmax, were determined using nonlinear least squares analysis with the software, FitAll, or alternatively were fitted by eye using Microsoft Excel (Redman, WA) v. 5.0 on a Macintosh computer (Cupertino, CA). The stoichiometry between factor VIII binding sites and vWF subunits was determined using fluorescein-labeled MoAb vW/16.9.2 as described under the Results.
For binding experiments with ΔPro-vWF, which had formed disulfide-linked oligomers under the influence of the cotransfected vWF propeptide, binding of factor VIII was not optimally fitted by equation 1. However, binding was well fitted by a modification of equation 1, which took into account two classes of binding sites with differing affinities for factor VIII:
where F1max and F2max refer to the fluorescence from saturating the higher and lower affinity binding sites with fluorescein-labeled factor VIII. The dissociation constants, KD1 and KD2, pertain to the higher and lower affinities of the two types of sites. Curve fitting of this data was performed by eye using Microsoft Excel v. 5.0 on a Macintosh computer.
Factor VIII binding to vWF in solution.
Fluorescein-labeled factor VIII (2 nmol/L) was incubated with increasing concentrations of vWF for 15 minutes at room temperature. Phospholipid bilayers supported by 2-μm diameter glass microspheres were added and, after 10 minutes, phospholipid-bound factor VIII was measured using flow cytometry. vWF-bound factor VIII was calculated as fraction of fluorescence competed from liposphere binding. Curve fitting was performed by eye using Microsoft Excel on a Macintosh computer. The equation used is in the initial description of this assay.3 The composition of the phospholipid bilayers was PS: phosphatidyl-choline: phosphatidyl-ethanolamine: cholesterol (4:70:26:10).
Western Blot Analysis
After measuring the concentration of vWF in the conditioned medium by ELISA, equal amounts of vWF in conditioned medium were electrophoresed on reducing SDS-polyacrylamide gels. Transfer of proteins to nitrocellulose filters was performed in 0.192 mol/L glycine and 0.025 mol/L Tris HCl as described.41 Nitrocellulose filters were blocked in PBS containing 2% Blotto42 and with 0.1% Tween 20. Anti-vWF antibody was added at 5 μg/mL in a solution of PBS with Blotto and 0.1% Tween 20. After several washes of the blot in PBS with 0.1%Tween 20, goat antirabbit secondary antibody was added at 0.15 μg/mL. After multiple washings, the filters were developed by ECL, and band intensities were quantified using the ImageQuant program and scanning hardware (Molecular Dynamics, Sunnyvale, CA).
Protein Sequence Analysis
COS-1 cells were transfected with the expression vector encoding ΔPro+A vWF. At 60 hours posttransfection, conditioned medium was harvested. Protein was immunoprecipitated with polyclonal anti-vWF antibody (The Binding Site, Birmingham, UK) and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Protein was electroblotted onto ProBlott membrane using CAPS buffer (10 mmol/L 3-[cyclohexylamino]-1-propanesulfonic acid, pH 11, 10% methanol) at 20 V overnight. The protein was detected with Coomassie Blue staining and excised from the membrane. Protein was sequenced by the University of Michigan protein sequencing core. The sequential recovery of residues for ΔPro+A-vWF was A (4 pmol/L), S (1 pmol/L), L (5 pmol/L), S (1 pmol/L), A (2 pmol/L), R (1 pmol/L), P (2 pmol/L), P (2 pmol/L), M (1 pmol/L), V (2 pmol/L), K (2 pmol/L), and L (4 pmol/L).
In Vitro vWF Propeptide Cleavage Analysis
Conditioned medium from PACE-Sol and mock-transfected cells were harvested using OptiMEM medium without protease inhibitors. This conditioned medium was then concentrated by centrifugation in Centricon 10 filters and dialyzed against 0.15 mol/L CO3Na2/HNaCO3, pH 6.5. Microtiter plates were coated with anti-vWF antibody (The Binding Site), as described above. After blocking the plates, equal amounts of vWF in conditioned medium were added to the plates and incubated at 37°C for 2 hours. The vWF-containing conditioned medium was removed and the plates were washed four times with 0.15 mol/L sodium acetate, pH 6.5. Equal volumes of PACE-Sol or mock-transfected cell conditioned medium in sodium acetate buffer with 5 mmol/L CaCl2 were added to the plates and incubated for 2 hours at 37°C. After washing plates in TBST buffer, purified recombinant factor VIII was added to the wells and incubated for an additional 2 hours. After washing, factor VIII binding was determined by factor VIII Coatest assay. A parallel plate was washed and SDS-PAGE sample buffer was added for subsequent SDS-PAGE and Western immunoblot analysis.
RESULTS
Multimeric vWF Has Higher Affinity for Factor VIII Than Dimeric vWF
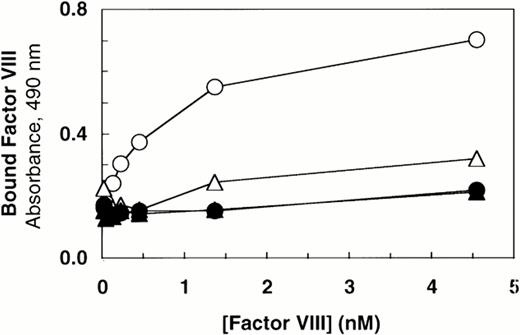
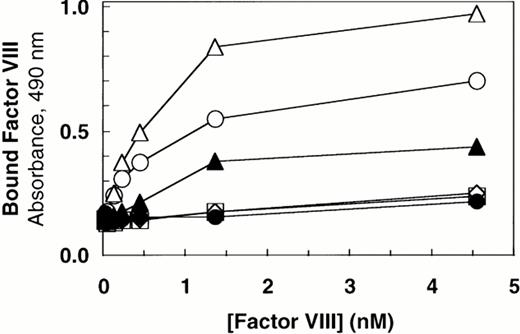
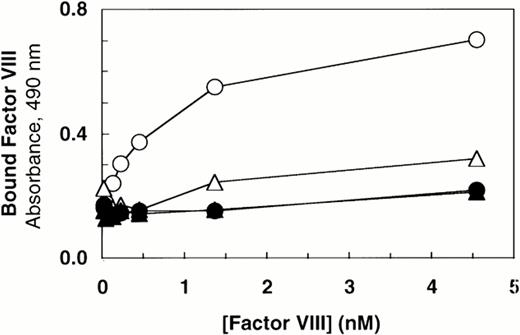
Deletion of the vWF propeptide yields dimeric vWF, the two subunits linked by C-terminal disulfide bonds. In the absence of the propeptide, the cysteine residues near the N-terminus of vWF subunits that ordinarily link dimers to form high molecular weight multimers are presumably not oxidized. To characterize the importance of intersubunit disulfide bonds that contribute to multimer formation, we compared binding of factor VIII to dimeric vWF (ΔPro-vWF) versus wild-type multimeric vWF. Recombinant vWF in conditioned medium from transfected COS-1 cells was quantitated by vWF ELISA and characterized by Western blot analysis. This analysis demonstrated that each construct expressed vWF at similar levels and that the mature vWF from the wild-type vWF expression vector comigrated with ΔPro-vWF (Fig 1, lanes 1 and 3). To measure factor VIII binding, equal concentrations of vWF in conditioned medium were incubated with increasing concentrations of recombinant factor VIII. The incubation mixtures were immobilized on microtiter plates with an anti-factor VIII heavy chain antibody (data not shown) or with anti-vWF antibody. Binding of factor VIII to ΔPro-vWF was substantially reduced by comparison to wild-type vWF, but significantly greater than that obtained from conditioned medium from mock-transfected cells (Fig 2). Similar results were obtained when complexes were captured with the factor VIII MoAb.
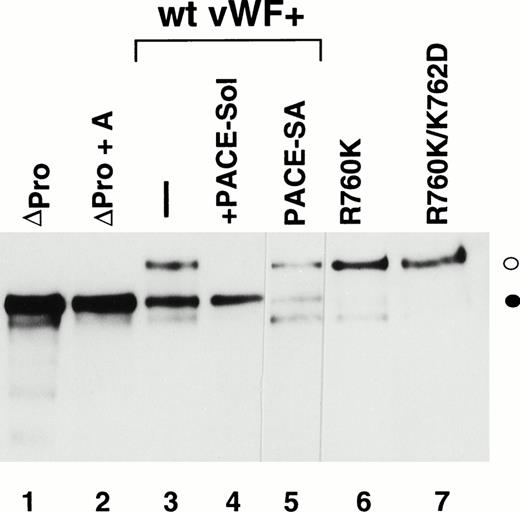
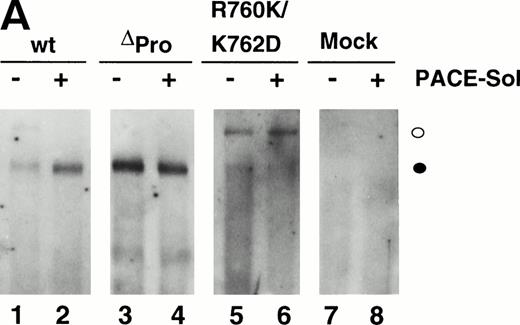
Western immunoblot analysis of vWF propeptide processing. Equal aliquots of conditioned medium from transiently transfected COS-1 cells were analyzed by SDS-PAGE under reducing conditions. Proteins were transferred to nitrocellulose and probed with anti-vWF antibody as described in the Materials and Methods. Where cotransfection was performed, equal amounts of the two plasmid DNAs were used in the transfection. The ratio of pro-vWF to mature was quantified by scanning as described in the Materials and Methods. The amount of pro-vWF in this analysis is underrepresented due to the poorer transfer efficiency of the high molecular weight pro-vWF. (○) Pro-vWF; (•) mature vWF.
Western immunoblot analysis of vWF propeptide processing. Equal aliquots of conditioned medium from transiently transfected COS-1 cells were analyzed by SDS-PAGE under reducing conditions. Proteins were transferred to nitrocellulose and probed with anti-vWF antibody as described in the Materials and Methods. Where cotransfection was performed, equal amounts of the two plasmid DNAs were used in the transfection. The ratio of pro-vWF to mature was quantified by scanning as described in the Materials and Methods. The amount of pro-vWF in this analysis is underrepresented due to the poorer transfer efficiency of the high molecular weight pro-vWF. (○) Pro-vWF; (•) mature vWF.
Factor VIII binding to vWF. Based on ELISA, equal amounts of vWF protein in the conditioned medium from transfected cells (100 ng/mL) were incubated with increasing concentrations of recombinant-derived factor VIII. The complexes were immobilized by capture using microtiter plates coated with anti-vWF polyclonal antibody. Bound proteins were quantified by factor VIII activity as described in the Materials and Methods. (○) Wild-type vWF; (▵) ΔPro-vWF; (▴) ΔPro+A-vWF; (•) conditioned medium from mock-transfected cells. Although these results represent single values from a single experiment in which protein was analyzed in parallel by Western blot analysis (Fig 1), these binding curves were performed at least 5 times from different transfection experiments and yielded data qualitatively similar to that reported here.
Factor VIII binding to vWF. Based on ELISA, equal amounts of vWF protein in the conditioned medium from transfected cells (100 ng/mL) were incubated with increasing concentrations of recombinant-derived factor VIII. The complexes were immobilized by capture using microtiter plates coated with anti-vWF polyclonal antibody. Bound proteins were quantified by factor VIII activity as described in the Materials and Methods. (○) Wild-type vWF; (▵) ΔPro-vWF; (▴) ΔPro+A-vWF; (•) conditioned medium from mock-transfected cells. Although these results represent single values from a single experiment in which protein was analyzed in parallel by Western blot analysis (Fig 1), these binding curves were performed at least 5 times from different transfection experiments and yielded data qualitatively similar to that reported here.
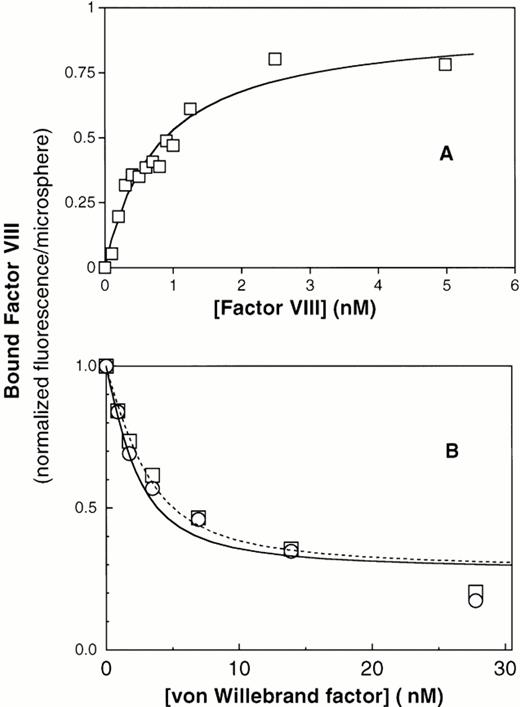
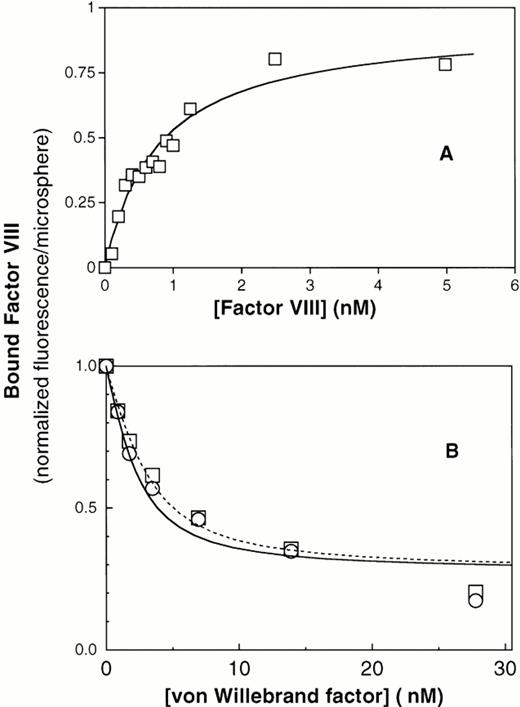
To investigate whether the reduced binding of factor VIII to ΔPro-vWF was due to a reduced affinity or loss of some binding sites, we developed a quantitative equilibrium binding assay. Our system was based on the recent observation that vWF captured by anti-vWF MoAbs on agarose beads binds factor VIII equivalently to vWF in solution.43 We activated cross-linked agarose beads of uniform size (Superose) with cyanogen bromide and coupled the noninhibitory anti-vWF MoAb vW/13.7.9 to the beads. In preliminary experiments, we found that ΔPro-vWF labeled with fluorescein-maleimide bound to the MoAb vW/13.7.9-Superose beads and that bound ΔPro-vWF did not dissociate more than 10% over 30 minutes (data not shown). Therefore, we concluded that ΔPro-vWF and vWF immobilized in this way were suitable for quantitative studies of factor VIII binding. Purified vWF was captured by the suspended MoAb vW/13.7.9-Superose beads, the beads were washed, and binding of fluorescein-conjugated factor VIII was investigated by flow cytometry. We found that factor VIII bound saturably to plasma vWF with a KD of 0.94 nmol/L (Fig 3A). To estimate the fraction of vWF binding sites available to factor VIII, we estimated the number of bound vWF subunits using fluorescein-labeled anti-vWF MoAb vW/16.9.2. By comparing the ratio of maximum fluorescence from bound MoAb vW/16.9.2 to fluorescence from bound fluorescein-labeled factor VIII, correcting from the fluorescein/protein ratio, we were able to compare the ratio of factor VIII binding sites with the number of vWF subunits recognized by MoAb vW/16.9.2. The results indicated a ratio of 0.99 (n = 2). Thus, the factor VIII binding sites are accessible to the same degree as an unrelated epitope.
Equilibrium binding of factor VIII to plasma vWF and to recombinant vWF. (A) Binding of fluorescein-labeled factor VIII to immobilized plasma vWF. Purified vWF from human plasma was immobilized onto MoAb vW/13.7.9-coated Superose-12 beads. After 1 hour of incubation at room temperature, increasing amounts of fluorescein-labeled factor VIII were added and incubated for 15 minutes. Fluorescence per bead was monitored by flow cytometry, as described under the Materials and Methods. The best fit curve corresponds to a KD of 0.8 nmol/L. (B) Displacement of factor VIII binding to PS-containing lipospheres by plasma vWF and recombinant vWF purified from CHO cells. (□) Plasma vWF; (○) recombinant vWF. In this assay, displacement of factor VIII from lipospheres does not reach zero, apparently because vWF binds with low affinity to lipospheres (data not shown). Therefore, the portion of the curve fitted correlates to displacement of 60% of factor VIII. The residual fluorescence, apparently not subject to displacement under these conditions, was excluded from quantitative analysis.
Equilibrium binding of factor VIII to plasma vWF and to recombinant vWF. (A) Binding of fluorescein-labeled factor VIII to immobilized plasma vWF. Purified vWF from human plasma was immobilized onto MoAb vW/13.7.9-coated Superose-12 beads. After 1 hour of incubation at room temperature, increasing amounts of fluorescein-labeled factor VIII were added and incubated for 15 minutes. Fluorescence per bead was monitored by flow cytometry, as described under the Materials and Methods. The best fit curve corresponds to a KD of 0.8 nmol/L. (B) Displacement of factor VIII binding to PS-containing lipospheres by plasma vWF and recombinant vWF purified from CHO cells. (□) Plasma vWF; (○) recombinant vWF. In this assay, displacement of factor VIII from lipospheres does not reach zero, apparently because vWF binds with low affinity to lipospheres (data not shown). Therefore, the portion of the curve fitted correlates to displacement of 60% of factor VIII. The residual fluorescence, apparently not subject to displacement under these conditions, was excluded from quantitative analysis.
To confirm that immobilization of vWF on MoAb vW/13.7.9-Superose beads did not influence binding of factor VIII and to verify that the production of vWF in cell culture (wild-type) does not affect its binding to factor VIII, binding of factor VIII to plasma vWF and wild-type vWF from CHO cells in solution was measured in competition binding assay. Because vWF and PS-containing membranes compete for the same factor VIII binding motif, we were able to detect binding of factor VIII to vWF in solution as decreased binding to PS-containing membranes supported by glass microspheres, as previously described.3 In this system, increasing vWF concentrations decreased membrane binding of factor VIII by 80% to 90% (Fig3B). The observed displacement for both vWF types was analyzed by fitting the data points to the equation cited in the Materials and Methods. The dissociation constants obtained under these conditions were 0.68 nmol/L and 1.05 nmol/L for plasma vWF and wild-type recombinant vWF, respectively (Table 1). These results indicate that binding of factor VIII to vWF is not influenced by posttranslational processing differences between endothelial cells and CHO cells. They also indicated that immobilization of vWF on MoAb vW/13.7.9 linked to Superose does not alter binding of factor VIII.
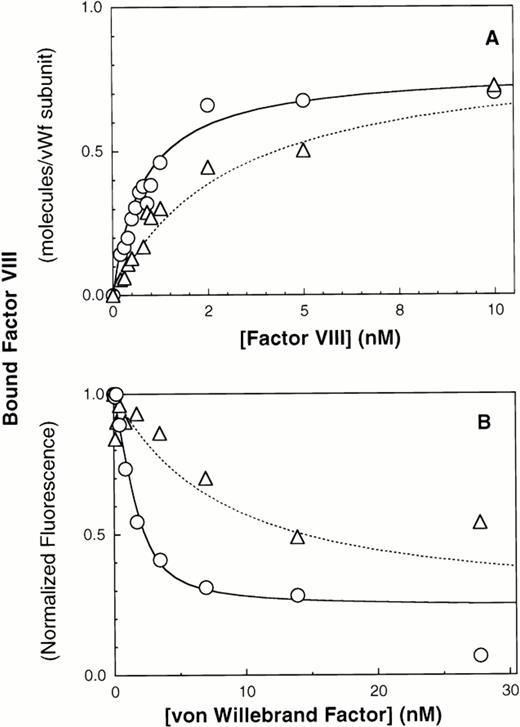
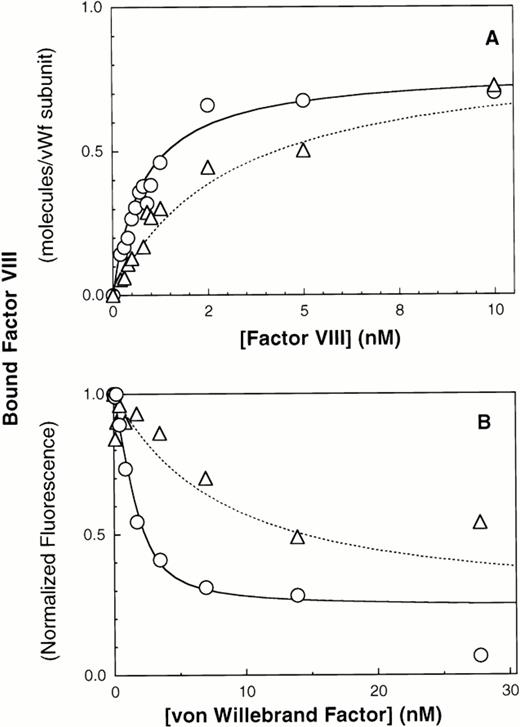
We next evaluated the importance of N-terminal intersubunit vWF disulfide bonds by immobilizing ΔPro-vWF onto MoAb vW/13.7.9 and compared the affinity of factor VIII for ΔPro-vWF versus plasma vWF. The results indicate that the affinity of factor VIII for ΔPro-vWF is lower than wild-type vWF. The best fit curves indicate that factor VIII binds to plasma vWF with a KD of 0.94 nmol/L versus 5 nmol/L for ΔPro-vWF (Figs 3A and 4A and Table 1). In these experiments, the number of vWF subunits immobilized on the microspheres was measured with fluorescein-labeled anti-vWF MoAb vW/16.9.2. The ratio between the number of factor VIII binding sites and the number of MoAb vW/16.9.2 epitopes detected was 0.99 for vWF and 1.37 for ΔPro-vWF (Table 1). Therefore, the intersubunit disulfide bonds predominately affect the affinity of factor VIII for vWF but not the stoichiometry between factor VIII and subunit binding sites.
Multimerization increases the affinity of vWF subunits for factor VIII. vWF purified from human plasma or dimeric mutant ΔPro-vWF, incapable of multimerizing and purified from CHO cells, were immobilized on MoAb vW/13.7.9-Superose-12 beads. Increasing concentrations of fluorescein-conjugated factor VIII were added and incubated for 15 minutes to allow equilibrium. Fluorescence per particle was monitored by flow cytometry and shown in (A). Parameters fitted were KD = 0.94 and 5 nmol/L for vWF and ΔPro-vWF, respectively. Binding of factor VIII to vWF in solution was measured in a competition binding assay and is shown in (B). Purified vWF or ΔPro-vWF, at various concentrations, was incubated with 2 nmol/L fluorescein-labeled factor VIII for 15 minutes at room temperature. Lipospheres were added and incubated for an additional 10 minutes and liposphere-bound fluorescein-labeled factor VIII was measured by flow cytometry. vWF-bound factor VIII was interpreted as the fraction not available for binding to lipospheres. The best-fit curves corresponds to the following parameter values: KD= 0.68 ± 0.28 (n = 3) and 6.4 ± 0.64 (n = 3) nmol/L and a stoichiometry of 0.9 and 0.8 factor VIII molecules per vWF subunit, for plasma vWF and Δpro-vWF, respectively. (○) vWF; (▵) Δpro-vWF.
Multimerization increases the affinity of vWF subunits for factor VIII. vWF purified from human plasma or dimeric mutant ΔPro-vWF, incapable of multimerizing and purified from CHO cells, were immobilized on MoAb vW/13.7.9-Superose-12 beads. Increasing concentrations of fluorescein-conjugated factor VIII were added and incubated for 15 minutes to allow equilibrium. Fluorescence per particle was monitored by flow cytometry and shown in (A). Parameters fitted were KD = 0.94 and 5 nmol/L for vWF and ΔPro-vWF, respectively. Binding of factor VIII to vWF in solution was measured in a competition binding assay and is shown in (B). Purified vWF or ΔPro-vWF, at various concentrations, was incubated with 2 nmol/L fluorescein-labeled factor VIII for 15 minutes at room temperature. Lipospheres were added and incubated for an additional 10 minutes and liposphere-bound fluorescein-labeled factor VIII was measured by flow cytometry. vWF-bound factor VIII was interpreted as the fraction not available for binding to lipospheres. The best-fit curves corresponds to the following parameter values: KD= 0.68 ± 0.28 (n = 3) and 6.4 ± 0.64 (n = 3) nmol/L and a stoichiometry of 0.9 and 0.8 factor VIII molecules per vWF subunit, for plasma vWF and Δpro-vWF, respectively. (○) vWF; (▵) Δpro-vWF.
Results obtained with immobilized vWF and ΔPro-vWF were confirmed using the competition experiment described above in Fig 3B. The dissociation constant for plasma vWF was 0.68 nmol/L and for ΔPro-vWF was 6.4 nmol/L (Fig 4B), whereas the binding stoichiometry between factor VIII and vWF subunits was 0.9:1 for both plasma vWF and ΔPro-vWF.
vWF Propeptide Coexpression Increases Factor VIII Binding to ΔPro-vWF
To further probe the possibility that multimer formation increases affinity of vWF subunits for factor VIII, we coexpressed the vWF propeptide with ΔPro-vWF. COS-1 cells were cotransfected with ΔPro-vWF and the vWF propeptide expression construct pMT2-KRX.15 By means of gel electrophoresis under nonreducing conditions, we confirmed that coexpression of the vWF propeptide with ΔPro-vWF yields a multimeric vWF (Fig 5A), as we have previously reported.15 We then immobilized the partially multimerized ΔPro-vWF to MoAb vW/13.7.9-Superose beads and added saturable amounts of fluorescein-labeled factor VIII, as described above. We observed saturable binding of factor VIII with an apparent affinity intermediate between plasma vWF and ΔPro-vWF (Fig 5B). These results confirm that formation of multimers increases the affinity of vWF subunits for factor VIII.
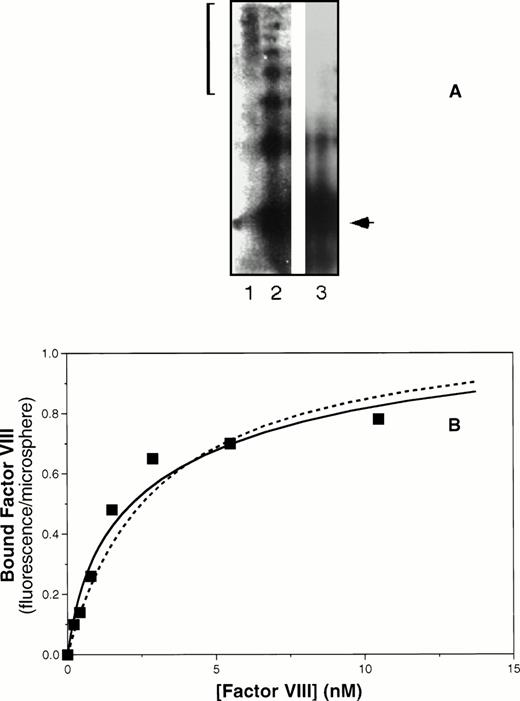
Coexpression of the vWF propeptide increases binding affinity of ΔPro-vWF for factor VIII. COS-1 cells were transfected with ΔPro-vWF in the presence or absence of the vWF propeptide expression vector pMT2-KRX. The resulting protein was evaluated by agarose gel electrophoresis under nonreducing conditions and transferred to nitrocellulose for detection as described in the Materials and Methods and shown in (A). Lane 1, plasma-derived vWF; lane 2, ΔPro-vWF coexpressed with the propeptide; lane 3, ΔPro-vWF without the propeptide. The bracket on the left indicates the distribution of vWF multimers in plasma. The arrow indicates the migration of the dimeric form of vWF. (B) Factor VIII binding as the fluorescence per Superose microsphere monitored by flow cytometry, measured as described for Fig 4. The solid fitted curve corresponds to a model with two classes of binding sites corresponding to lower affinity sites on non–disulfide-linked N-termini and higher affinity sites on disulfide-linked N-termini. The corresponding KDs were 0.77 and 6.5 nmol/L. The dashed curve corresponds to the same maximum fluorescence/microsphere but a single class of binding sites with intermediate affinity, KD of 3 nmol/L.
Coexpression of the vWF propeptide increases binding affinity of ΔPro-vWF for factor VIII. COS-1 cells were transfected with ΔPro-vWF in the presence or absence of the vWF propeptide expression vector pMT2-KRX. The resulting protein was evaluated by agarose gel electrophoresis under nonreducing conditions and transferred to nitrocellulose for detection as described in the Materials and Methods and shown in (A). Lane 1, plasma-derived vWF; lane 2, ΔPro-vWF coexpressed with the propeptide; lane 3, ΔPro-vWF without the propeptide. The bracket on the left indicates the distribution of vWF multimers in plasma. The arrow indicates the migration of the dimeric form of vWF. (B) Factor VIII binding as the fluorescence per Superose microsphere monitored by flow cytometry, measured as described for Fig 4. The solid fitted curve corresponds to a model with two classes of binding sites corresponding to lower affinity sites on non–disulfide-linked N-termini and higher affinity sites on disulfide-linked N-termini. The corresponding KDs were 0.77 and 6.5 nmol/L. The dashed curve corresponds to the same maximum fluorescence/microsphere but a single class of binding sites with intermediate affinity, KD of 3 nmol/L.
Inspection of the nonreducing gel indicated that, although the propeptide had induced vWF multimer formation, a significant fraction of vWF remained as dimers (Fig 5A, lane 2). The dimers are predicted to bind factor VIII with lower affinity. In addition, each higher molecular weight multimer is predicted to have two terminal vWF subunits with low affinity for factor VIII in addition to the internal subunits with high affinity for factor VIII. We used this reasoning, together with the measured quantity of vWF of various multimer sizes determined by laser densitometry, to predict that the partially multimerized vWF in lane 2 of Fig 5A would contain 42% high-affinity sites and 58% low-affinity sites. The solid curve depicted in Fig 5B was fit to the data using a model with two classes of binding sites. The KDs corresponding to the curve were 0.77 and 6.5 nmol/L. The proportion of low-affinity sites was 64%, with 36% higher-affinity binding sites. Although the data may also be fitted for a single class of binding sites of intermediate affinity (KD = 3 nmol/L, dashed curve, Fig 5B), the fit is less good and we lack a rationale for predicting formation of actual intermediate-affinity binding sites. Thus, these data are consistent with the interpretation that formation of N-terminal disulfide-linked multimer bonds increases the affinity of factor VIII binding sites.
vWF Propeptide Cleavage and a Native Mature N-Terminus Is Required for Factor VIII Binding
The results in this report indicate that each vWF subunit in a ΔPro-vWF dimer contains one factor VIII binding site with approximately sixfold lower affinity than the internal factor VIII binding sites on vWF multimers. In contrast, a prior report suggested that binding of factor VIII to vWF was abolished on vWF dimers secreted in the absence of the propeptide.35 We hypothesized that this apparent discrepancy was due to one extra Ala residue at the junction of the signal peptide and the mature vWF polypeptide in the propeptide-deletion construct prepared in the latter study. To evaluate the importance of the addition of this Ala for factor VIII binding, we expressed that construct, ΔPro+A-vWF, in parallel with ΔPro-vWF and compared binding of factor VIII. Under conditions in which binding to ΔPro-vWF was detected, binding to ΔPro+A-vWF was not detected (Fig2). We also evaluated binding of ΔPro+A-vWF using the equilibrium binding assay. Successful capture of ΔPro+A-vWF was demonstrated with fluorescein-labeled MoAb vW/16.9.2. However, no binding of factor VIII was detected (data not shown). The difference in the ability for ΔPro-vWF and ΔPro+A-vWF to bind factor VIII supported our hypothesis and indicated that minor perturbations at the vWF N-terminus may decrease affinity for factor VIII by more than 20-fold.
ΔPro-vWF and ΔPro+A-vWF differ by the presence of a single alanine residue at the junction of the signal peptide sequence and the mature sequence in ΔPro+A-vWF. Because the addition of this residue could alter the site of signal peptide processing, we determined the N-terminal sequence of mature secreted ΔPro+A-vWF to identify the site of signal peptide cleavage. Conditioned medium was harvested from ΔPro+A-vWF–transfected cells and immunoprecipitated for SDS-PAGE after reduction. The polypeptide migrating at the position of monomeric vWF was isolated. The sequence was determined to contain an N-terminal Ala immediately upstream from the N-terminal 10 residues of mature vWF (see the Materials and Methods).
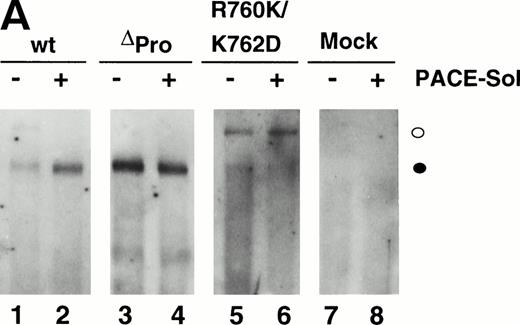
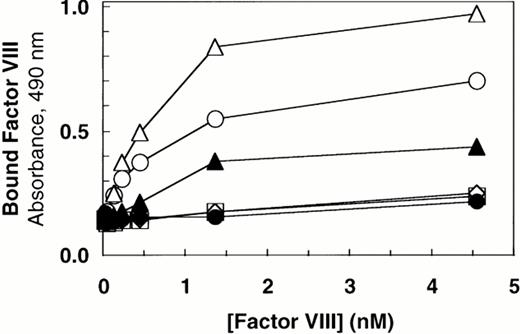
We previously reported that a mutant form of vWF in which the propeptide was not cleaved during intracellular processing did not bind factor VIII.34 In that construct, the P1 position with respect to the vWF propeptide cleavage site, Arg763, was mutated to Lys. Because factor VIII binding may be perturbed by small alterations in this region, such as the presence of an extra alanine at the N-terminus of mature vWF, we were concerned that the failure of the R763K mutant to bind factor VIII resulted from the amino acid substitution at residue 763 rather than the attached propeptide.34 To further evaluate the importance of the vWF propeptide cleavage for factor VIII binding, we studied the factor VIII binding of two different pro-vWF propeptide cleavage site mutants. Western blot analysis of the single mutant R760K or the double mutant R760K/K762D demonstrated that the propeptides of these mutants were not detectably processed by the endogenous COS-1 processing enzyme (Fig 1, lanes 6 and 7). Analysis of factor VIII binding indicated that both mutants were defective in binding factor VIII (Fig 6). The defective factor VIII binding observed for these noncleavage mutants supports the conclusion that propeptide cleavage is required for factor VIII binding.
The presence of the vWF propeptide inhibits factor VIII binding. COS-1 cells were transfected with wild-type and propeptide cleavage site mutant (R760K and R760K/K762D) vWF, as well as with the expression vectors encoding wild-type vWF in the absence or presence of PACE-Sol or a catalytically inactive mutant PACE-SA. Conditioned medium was harvested for factor VIII binding assays and Western immunoblot analysis of vWF (Fig 1). The reduced amount of vWF detected upon PACE-SA cotransfection was reproducible and was corrected for in the factor VIII binding assay by using increased amounts of conditioned medium for this sample. The concentration of vWF in the conditioned medium was determined by ELISA and the binding assays were performed using equal amounts of vWF and were measured by factor VIII activity assay as described in the Materials and Methods. (○) Wild-type vWF; (□) R760K vWF; (◊) R760K/K762D vWF; (▵) vWF+PACE-Sol; (▴) vWF+PACE-SA; (•) mock.
The presence of the vWF propeptide inhibits factor VIII binding. COS-1 cells were transfected with wild-type and propeptide cleavage site mutant (R760K and R760K/K762D) vWF, as well as with the expression vectors encoding wild-type vWF in the absence or presence of PACE-Sol or a catalytically inactive mutant PACE-SA. Conditioned medium was harvested for factor VIII binding assays and Western immunoblot analysis of vWF (Fig 1). The reduced amount of vWF detected upon PACE-SA cotransfection was reproducible and was corrected for in the factor VIII binding assay by using increased amounts of conditioned medium for this sample. The concentration of vWF in the conditioned medium was determined by ELISA and the binding assays were performed using equal amounts of vWF and were measured by factor VIII activity assay as described in the Materials and Methods. (○) Wild-type vWF; (□) R760K vWF; (◊) R760K/K762D vWF; (▵) vWF+PACE-Sol; (▴) vWF+PACE-SA; (•) mock.
To further demonstrate the requirement for vWF propeptide cleavage within wild-type pro-vWF in factor VIII binding, we modulated cleavage of wild-type pro-vWF that occurred by coexpression of PACE-Sol (a soluble secreted form of PACE/furin) that was previously shown to improve vWF processing39 or by coexpression of a catalytically inactive serine to alanine active site mutant of PACE/furin (PACE-SA) that was previously shown to inhibit pro-vWF processing.39 Analysis of vWF processing by Western blot analysis of conditioned medium indicated that processing of wild-type pro-vWF expressed alone was incomplete, whereas one third of the secreted vWF was in the precursor form (Fig 1, lane 3), similar to previous observations.23 24 Upon cotransfection with PACE-Sol, all the secreted vWF was in the mature form (Fig 1, lane 4). In contrast, coexpression with the PACE-SA mutant yielded at least 60% of the vWF precursor form as pro-vWF (Fig 1, lane 5). Factor VIII binding assays performed on the vWF conditioned medium demonstrated that cotransfection of PACE-Sol increased factor VIII binding to wild-type vWF (Fig 6). In contrast, cotransfection with mutant PACE-SA reduced the binding to factor VIII (Fig 6). As a control, cotransfection of PACE-Sol with ΔPro-vWF did not affect the binding of ΔPro-vWF to factor VIII (data not shown). These results correlate the degree of vWF propeptide processing with the degree of factor VIII binding to wild-type vWF.
In Vitro Propeptide Cleavage Increases Factor VIII Binding
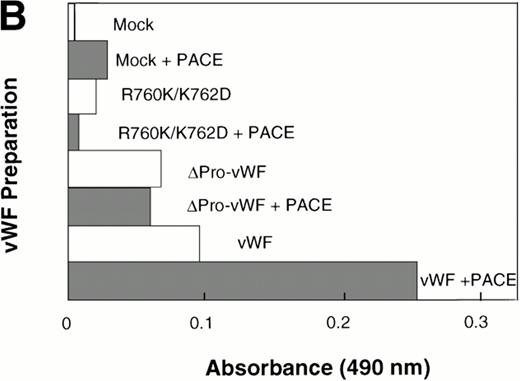
The ability for PACE-Sol to cleave the vWF propeptide in vitro was tested by immobilizing wild-type vWF from COS-1 transfected cells onto microtiter plates. Subsequently, conditioned medium from PACE-Sol–transfected or mock-transfected COS-1 cells was incubated with the samples and cleavage was monitored by Western immunoblotting analysis using anti-vWF antibody. The results demonstrated that incubation with PACE-Sol increased propeptide cleavage to 100% in this cell-free system (Fig 7A, lanes 1 and 2). Incubation of PACE-Sol with either ΔPro-vWF or the propeptide cleavage site mutant R760K/K762D had no effect (Fig 7A, lanes 3 through 6). These results support that cleavage occurred at the authentic propeptide cleavage site. These results show that PACE-Sol can mediate propeptide processing of pro-vWF in a cell-free system.
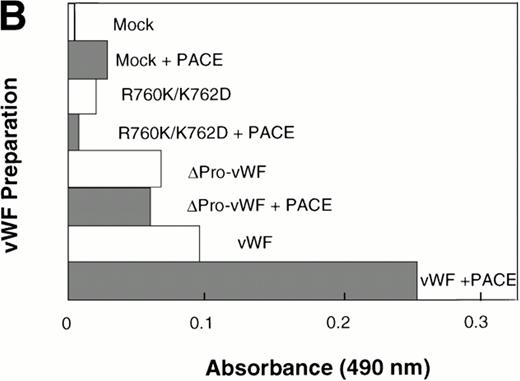
In vitro cleavage of pro-vWF by PACE/furinincreases binding affinity to factor VIII. COS-1 cells were transfected with wild-type vWF, ΔPro-vWF, or cleavage site mutant R760K/K762D vWF and conditioned medium was harvested. vWF from the conditioned medium was immobilized on anti-vWF polyclonal antibody-coated microtiter plates. The immobilized vWF was then treated with concentrated conditioned medium from PACE-Sol–transfected or mock-transfected COS-1 cells as described in the Materials and Methods. In (A), proteins were recovered from the wells and analyzed by SDS-PAGE under reducing conditions and Western blot analysis using polyclonal anti-vWF antibody. (○) Pro-vWF; (•) mature vWF. (B) Factor VIII binding (1.25 nmol/L) to vWF preparations (wild-type, ΔPro-vWF and R760K/K762D vWF) that were immobilized on anti-vWF polyclonal antibody-coated microtiter plates and then treated with conditioned medium from mock-transfected or PACE-Sol-transfected (+ PACE) COS-1 cells. Although the results depicted here are from a single experiment with a single concentration of factor VIII in which Western blot analysis was performed in parallel (A), this experiment was performed several times with varying concentrations of factor VIII and gave consistent results.
In vitro cleavage of pro-vWF by PACE/furinincreases binding affinity to factor VIII. COS-1 cells were transfected with wild-type vWF, ΔPro-vWF, or cleavage site mutant R760K/K762D vWF and conditioned medium was harvested. vWF from the conditioned medium was immobilized on anti-vWF polyclonal antibody-coated microtiter plates. The immobilized vWF was then treated with concentrated conditioned medium from PACE-Sol–transfected or mock-transfected COS-1 cells as described in the Materials and Methods. In (A), proteins were recovered from the wells and analyzed by SDS-PAGE under reducing conditions and Western blot analysis using polyclonal anti-vWF antibody. (○) Pro-vWF; (•) mature vWF. (B) Factor VIII binding (1.25 nmol/L) to vWF preparations (wild-type, ΔPro-vWF and R760K/K762D vWF) that were immobilized on anti-vWF polyclonal antibody-coated microtiter plates and then treated with conditioned medium from mock-transfected or PACE-Sol-transfected (+ PACE) COS-1 cells. Although the results depicted here are from a single experiment with a single concentration of factor VIII in which Western blot analysis was performed in parallel (A), this experiment was performed several times with varying concentrations of factor VIII and gave consistent results.
We next asked whether in vitro cleavage of the vWF propeptide affects factor VIII binding. Under these in vitro conditions, it is unlikely that disulfide bond rearrangements occur; thus, any change in factor VIII binding affinity could be attributed only to cleavage of the propeptide. After in vitro cleavage of the propeptide, wild-type vWF binding to factor VIII increased approximately 2.5-fold (Fig 7B). For control, PACE-Sol conditioned medium was incubated with ΔPro-vWF or with the vWF cleavage site mutant R760K/K762D. PACE-Sol did not increase the binding of factor VIII to either of these vWF mutants (Fig7B). These results show that in vitro cleavage of the propeptide increases vWF binding to factor VIII.
DISCUSSION
The data in this report elucidate the importance of two vWF processing events that occur late in the biosynthetic pathway, propeptide cleavage and N-terminal disulfide bond formation. Removal of the vWF propeptide is necessary for factor VIII to interact with its binding site in the D′ domain of vWF. Because the addition of a single alanine to the mature N-terminus, like the presence of the large propeptide, abolished binding of factor VIII, the native N-terminus of vWF is necessary for factor VIII binding. These results are consistent either with direct interaction of the N-terminal Ser residue of vWF with factor VIII or with the native N-terminal Ser residue being required for correct folding of the N-terminus. The affinity of factor VIII for vWF binding sites is enhanced approximately sixfold upon vWF multimer formation via disulfide bonds, a change likely reflecting a conformational change of the D′ domain. These data imply that, in plasma, factor VIII is not bound to vWF subunits with attached propeptides and that factor VIII is far more likely to be found on internal vWF subunits than those that terminate the high molecular weight multimers.
Our studies have now resolved the previous conflicting results concerning the role of the vWF propeptide in binding to factor VIII.34,35 The results demonstrated that ΔPro-vWF, previously described by Wise et al,34 bound factor VIII but at a sixfold lower affinity than wild-type vWF. In contrast, ΔPro+A vWF, described by Leyte et al,35 did not bind factor VIII at all concentrations tested. Previous protein sequence analysis confirmed the correct N-terminus for the ΔPro-vWF deletion molecule.15 We have now confirmed that the secreted ΔPro+A contains an additional Ala at the N-terminus of the secreted vWF molecule and believe that inhibition of binding by the additional alanine is the only likely explanation for failure of factor VIII to bind to the dimeric vWF.
Previous studies demonstrated that the vWF propeptide can also act in trans to mediate multimerization of ΔPro-vWF.15 We have shown by cotransfection of a vWF propeptide expression vector with a ΔPro-vWF expression vector that the resulting multimers possess increased factor VIII binding. The propeptide sequence contains vicinal cysteine residues (2 cysteine residues separated by 2 amino acids) that are similar to the catalytic active sites of protein disulfide isomerase,44 which facilitates disulfide bond formation and exchange in the endoplasmic reticulum. In vivo, the vWF propeptide promotes disulfide bond formation between N-terminal ends and subsequently the propeptide is cleaved to generate the mature vWF N-terminus.21 In the present study, we conducted equilibrium binding studies by flow cytometry to demonstrate multimeric vWF prepared by coexpression of propeptide-deleted vWF with the propeptide displays increased binding affinity to factor VIII. When the binding data are corrected for the fraction of sites on disulfide-linked terminal vWF subunits, these multimers have the same affinity for factor VIII as plasma vWF (Fig 5B). There are two mechanisms by which the propeptide could increase the factor VIII binding affinity. First, the process of multimer formation may juxtapose two factor VIII binding sites that may increase affinity through proximity so that a single factor VIII molecule might interact with two adjacent vWF subunits. Second, multimer formation may cause a conformational change in the factor VIII binding motif of each vWF subunit. We prefer the second explanation, noting that the relatively large factor VIII binding motif spanning vWF amino acid residues 1-102 and the corresponding vWF binding motifs of factor VIII, separated by 600 amino acids in linear sequence,45-47 suggest a relatively large interacting surface where modest geometric changes could alter affinity. Alternatively, the propeptide may mediate disulfide bond exchange reactions within the N-terminal end of the mature vWF subunit to change the conformation of vWF to create a high-affinity factor VIII binding site. However, we think this latter possibility is unlikely because proteolytic fragments of plasma-derived vWF exhibit similar affinities to factor VIII, as observed for ΔPro-vWF,48 suggesting that disulfide exchanges may not be responsible for the difference between high-affinity and low-affinity sites.
In summary, these results indicate that propeptide-induced formation of N-terminal disulfide bonds between vWF dimers is necessary for formation of the highest affinity factor VIII binding sites. However, these sites remain cryptic until the propeptide is cleaved, eliminating steric hindrance or allowing correct folding of the vWF N-terminus.
ACKNOWLEDGMENT
The authors thank Dr J. van Mourik for kindly providing the vWFdelpro expression plasmid for these studies, K.C. Kye for assistance in developing the microtiter plate binding assays used in this study, and Dr Steven Pipe for critical review of this report.
Supported in part by National Institutes of Health Grants No. HL42443, HL53777, and HL52173; by the Medical Research Service of the Veterans Administration; and by a Grant in Aid from the American Heart Association (to R.J.W.). A.V.B. was supported by individual NRSA No. HL09506 and G.E.G. was a recipient of AHA Established Investigator Award No. 96001720.
Address reprint requests to Randal J. Kaufman, PhD, Howard Hughes Medical Institute, MSRBII, 1150 W Medical Center Dr, University of Michigan, Ann Arbor, MI 48109; e-mail: kaufmanr@umich.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.