To the Editor:
We read with interest the report of Wlodarski et al1published in a recent issue of Blood concerning the role of p53 in the recovery of hematopoieis after cytotoxic treatment. The investigators used as an experimental model p53 wild-type and p53 knock-out mice treated with 5-fluorouracil (5-FU). They showed that bone marrow (BM) mononuclear cells recovering in p53 knock-out mice contain a higher number of both primitive and multipotential progenitors than BM cells recovering in p53 wild-type mice. Moreover, p53 knock-out progenitors are responsible for the long-term engraftment of hematopoiesis when transplanted in lethally irradiated recipients. Thus, these data undoubtedly indicate that p53 is involved in the induction of apoptosis of hematopoietic progenitors previously exposed to DNA-damaging drugs.
To our knowledge, no data have yet been reported about the modulation of the tumor-suppressor gene p53 or of its target genes in human hematopoietic cells after in vivo chemotherapy administration. To this purpose, we used flow cytometry to evaluate the expression of p53 and of two apoptosis-related genes, bcl-2 and bax, in CD34+ cells recovering after chemotherapy. We evaluated 5 patients affected by non-Hodgkin’s lymphomas, candidates for peripheral blood (PB) stem cell collection and transplantation. All patients received cyclophosphamide (7 g/m2) and, from day 2 after chemotherapy, granulocyte colony-stimulating factor (G-CSF; 5 μg/kg). In all patients, BM and PB samples were collected on day 12 after chemotherapy. Data obtained in patients recovering from chemotherapy were then compared with those obtained in CD34+ cells isolated from normal BM and PB. All of the samples were collected after informed consent was obtained.
CD34+ cells were isolated from mononuclear cell fractions by the miniMACS starting kit and the CD34 multisort kit (Miltenyi Biotec Inc, Auburn, CA), according to the manufacturer. The purity of immunoselected CD34+ cells was evaluated as previously reported,2 and in all cases exceeded 90%. For the measurement of intracellular antigens, immunoselected CD34+cells were fixed and permeabilized by the Ortho Permeafix TM solution (Ortho Diagnostic System, Raritan, NJ); cells were then incubated with the following unconjugated monoclonal antibodies (MoAbs): anti–wild-type p53 (Ab1 clone; Calbiochem, Cambridge, MA) and anti–Bcl-2 (Ab2 clone; Calbiochem) or with the anti-Bax rabbit polyclonal antibody (P-19 clone; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 4°C. Control samples were incubated in the same experimental conditions with isotype-matched irrelevant unconjugated MoAb or with rabbit preimmune serum. After washing, cells were further incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse MoAb (Ortho) or with FITC-conjugated goat antirabbit Igs (Dako A/S, Glostrup, Denmark) for 1 hour at 4°C, at 1:20 and 1:1,000 final dilution, respectively. All samples were analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) equipped with an argon laser emitting at 488 nm. Results were expressed as the mean fluorescence intensity ratio (MFI-R), which is calculated as the ratio between the MFI of test curve and the MFI of control curve.
In addition, CD34+ cell samples were evaluated for the content of apoptotic cells by in situ end-labeling of DNA strand breaks with the TdT-mediated biotin-nick-end-labeling (TUNEL), as previously described.3 The slides were counterstained with hematoxylin and 100 consecutive cells in 3 or more fields were counted. Three different experiments were performed. The statistical analysis of data obtained was performed using the Mann Whitney test for unpaired data.
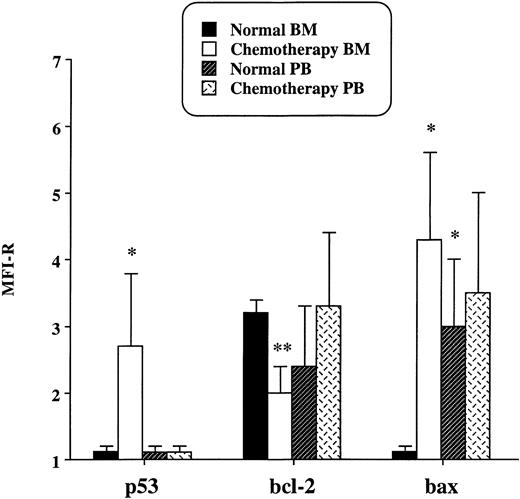
Results of flow cytometry analysis are shown in Fig1. It emerged that p53, undetectable in normal BM and PB CD34+ cells, was expressed in BM but not in PB progenitors recovering after chemotherapy. Moreover, in BM progenitors, the induction of p53 was accompained by a significant reduction of the expression of Bcl-2 and by the appearance of Bax. On the contrary, in PB CD34+ cells recovering after chemotherapy, no differences in the protein levels of Bcl-2 and Bax were detected when compared with normal PB CD34+ cells. In addition, in normal PB CD34+ progenitors, Bax was expressed at a higher level than in normal BM CD34+progenitors, but its expression did not further increase after chemotherapy. The upregulation of Bax has been reported in progenitor B cells detached from the stromal layer.4 Similarly, we can hypothesize that Bax is expressed in CD34+ progenitors released from the BM microenvironment to the PB. Data obtained by flow cytometry were consistant with the content of apoptotic cells evaluated by the TUNEL method (Table1). Actually, BM CD34+ cells recovering after chemotherapy showed a percentage of apoptotic cells significantly higher than that observed in the other samples evaluated. On the contrary, PB CD34+ progenitors recovering after chemotherapy showed few apoptotic cells. Finally, according to their increased Bax expression, normal PB CD34+ cells showed a higher content of apoptotic cells than did normal BM progenitors and PB progenitors isolated after chemotherapy.
Expression of p53, Bcl-2, and Bax in CD34+cells isolated from BM and PB of normal individuals and of patients treated with cyclophosphamide. Results are expressed as the MFI-R (mean value ± SD) of five experiments.
Expression of p53, Bcl-2, and Bax in CD34+cells isolated from BM and PB of normal individuals and of patients treated with cyclophosphamide. Results are expressed as the MFI-R (mean value ± SD) of five experiments.
Our observations agree with those of Wlodarski et al1 and confirm that p53 is induced in vivo in hematopoietic CD34+cells after chemotherapy. Moreover, the differences that we found between BM and PB CD34+ cells suggest that hematopoietic progenitors that recover from the cytostatic damage (p53 and Bax negative) leave BM, whereas those still suffering from the toxic insult (p53 and Bax positive) are retained in the BM microenvironment. Interestingly, Wlodarski et al1 reported in BM cells from p53 knock-out mice an increased expression of cyclin-dependent kinases inhibitor (CDK-I) p16INK4A and suggested that other regulatory mechanisms involving this CDK-I may compensate for the loss of p53. In a previous study, we demonstrated that the cell cycle arrest of CD34+ progenitors mobilized by chemotherapy is associated with the expression of the CDK-I p15INK4B.5 Thus, these observations suggest that p53 and CDK-Is (p15INK4B or p16INK4A) operate differently depending on whether cells are induced to or recover from apoptosis, respectively.
In conclusion, our data further support the possibility that hematopoietic recovery could be improved by interfering with the expression of p53 gene.
ACKNOWLEDGMENT
This work has been supported by a grant from A.I.R.C. (Associazione Italiana per la Ricerca sul Cancro).