Abstract
Neutrophils must adhere to the vessel wall, migrate, and degranulate in an ordered manner to perform their protective function. Disruption of these processes may be pathogenic. Current knowledge of the degranulation process is derived almost exclusively from studies on neutrophils in suspension, in which priming with the nonphysiological agent cytochalasin B is necessary to obtain elastase release in response to activating agents. To avoid this, we have adopted a different approach. Using a novel flow-based adhesion system, we have been able to quantify the release of elastase from the primary granules of activated neutrophils adherent to immobilized platelets or purified receptors without priming. Comparing stimuli, formyl tripeptide (fMLP), interleukin-8 (IL-8), activated complement fragment C5a, and platelet-activating factor (PAF) all induced rapid conversion to CD11b/CD18 (MAC-1) -mediated stationary adhesion when perfused over neutrophils already rolling on platelet monolayers or purified P-selectin. However, fMLP, C5a, and IL-8, but not PAF, induced release of elastase from the adherent cells in minutes. Neutrophils stimulated in suspension showed little degranulation. Treatment of neutrophils with an inhibitor of 5-lipoxygenase–activating protein (MK886) and thus synthesis of leukotrienes (LTs) or with an antagonist of the LTB4 receptor (LY223982) blocked the release of elastase. This indicated that endogenous synthesis of 5-lipoxygenase products such as LTs and autocrine activation of neutrophils was required for fMLP-driven elastase release. We hypothesize that the differential ability of PAF and fMLP to induce elastase release from surface-adherent neutrophils could arise from differential ability to generate leukotrienes, such as LTB4, and would be an appropriate mechanism for the control of elastase release during inflammation in vivo, where it is important that cytotoxic agents are not released until activated neutrophils have migrated into the extravascular tissues.
NEUTROPHILS MUST exit the circulation and enter inflamed tissues to undertake phagocytosis. Invasive micro-organisms are then destroyed through production of reactive oxygen species (ROS) and release of degradative enzymes from preformed granule stores. Granule enzymes, including elastase, are released not only intracellularly into phagosomes, but also into the extracellular environment, where they may have digestive roles.1,2Ordinarily neutrophils (and their products) are localized to an inflammatory locus by a series of adhesive and activating steps that are closely regulated by endothelial cells lining the microvasculature (for review, see Zimmerman et al,3 Imhof and Dunon,4 and Springer5). Clearly, regulation must extend to the control of neutrophil granule release during recruitment from the blood. Indeed, disregulation of the recruitment process can lead to inappropriate adhesion, ROS production, and degranulation of neutrophils, which is implicated in the pathologies of emphysema,6 venous disease,7 rheumatoid arthritis,8 organ failure in sepsis,9 and reperfusion of ischemic tissue (for review, see Welbourne et al10), including the heart, during myocardial infarction (for review see Siminiak and Ozawa11). The mechanisms controlling these responses are thus of great importance.
Products secreted by neutrophils are compartmentalized into at least three distinct granule populations. Primary granules contain elastase, myeloperoxidase, and lipases,1 which are generally associated with tissue remodelling in inflammation and tissue damage in pathology.1,2,6-11 Both secondary and tertiary granules contain a number of metaloproteinases,1 which specifically degrade the proteins found in extracellular matrix (eg, collagenase) and may be required for efficient transit of neutrophils from vessel lumen, through subendothelial matrix, and into tissues. Tertiary granules also contain the preformed pool of the β2-integrin heterodimer CD11b/CD18,2 which must be rapidly mobilized upon neutrophil activation to allow prolonged adhesion and migration.12-14 This raises the question of how the undesirable release of primary granule contents is separated from the secondary and tertiary granule mobilization required for the early stages of attachment to the vessel wall and for migration.
In vitro studies have shown that agents such as bacterial peptide analogues (formyl tripeptide [fMLP]), activated complement fragment C5a, platelet-activating factor (PAF), the chemokine interleukin-8 (IL-8), and the eicosanoid, leukotriene B4(LTB4), can promote rapid release of all neutrophil granular stores in suspension when the cells have been pretreated with the cytoskeleton-disrupting agent cytochalasin B.1,15,16However, cytochalasin B may not be essential for the release of secondary and tertiary granules17 and is not required for de novo expression of CD11b/CD18. Thus, only primary granule release may be mediated by a route requiring cytochalasin B in suspension. Although such differential responses are suggestive regarding a mechanism by which primary granule mobilization is separated from the mobilization of other granule compartments, modelling the processes in suspension is far removed from the physiological situation where neutrophils are activated at the vessel wall. A previous study using adherent neutrophils indicated that binding of CD11b/CD18 to albumin was sufficient to allow the release of primary and secondary granules in response to fMLP.18 Studies of flowing blood have also demonstrated that the release of elastase from activated neutrophils in the extracorporeal circulation during coronary bypass surgery19 or during hemodialysis20 correlated with the expression of β2-integrin or required adhesion via this receptor. However, detailed studies of the effect of adhesion on neutrophil degranulation in circulatory models have not been reported. Such studies might provide a better understanding of the pathophysiology of degranulation and of the mechanisms that control its localization.
We describe here a novel perfusion system that has allowed us to study adhesion and primary granule release of neutrophils independent of cytochalasin B priming. Flowing neutrophils formed rolling attachments to P-selectin presented on a monolayer of activated platelets or immobilized as a purified protein. Rolling cells were subsequently superfused with activating agents so that they converted to stationary adhesion via β2-integrin CD11b/CD18.14 21When perfusate was collected and assayed for content of elastase, it was evident that fMLP, C5a, and IL-8 caused adherent neutrophils to release this enzyme, but that PAF did not. The flow system allowed analysis of the kinetics of elastase release, which peaked within 2.5 minutes and then steadily decreased. We also found that endogenous synthesis of LTB4 via the 5-lipoxygenase pathway was an essential autocrine activation step for elastase release stimulated by fMLP under these conditions. Thus, adhesion to substrate can remove the requirement for cytochalasin B during induction of primary granule release by chemotactic agents, and differential induction by activating signals could be important in localizing the potentially pathological aspects of neutrophil degranulation to the extravascular space.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs).
MoAbs against CD18 were R6.5E (kind gift of Martyn Robinson, Celltech, Slough, UK), used at 24 μg/mL. Antibody against CD11b was KIM247 (gift of Martyn Robinson), used at 14 μg/mL. MoAb G1 against P-selectin (kind gift of Rodger McEver, University of Oklahoma, Oklahoma City, OK) was used at a concentration of 50 μg/mL. All antibodies were IgG1.
Isolation of neutrophils.
Blood was collected from healthy volunteers into citrate phosphate dextrose adenine-1 (CPDA-1; Baxter Health Care Ltd, Thetford, UK) and neutrophils were isolated using two-step density gradients of Histopaque (Sigma Chemical, Poole, UK), as previously described.14 22 Neutrophils were washed, counted using a Coulter counter (Coulter Electronics Ltd, Luton, UK), and adjusted to 1 × 106/mL in phosphate-buffered saline containing 1 mmol/L Ca2+ and 0.5 mmol/L Mg2+(PBS; Sigma) and 0.15% bovine serum albumin (BSA fraction IV; Sigma) (PBS/BSA).
Immobilization of platelet monolayers or P-selectin in microslides.
Microslides (CamLab, Cambridge, UK) are glass capillary tubes with a rectangular cross-section of 0.3 × 3 mm, a length of 5 cm, and good optical qualities. To provide a substrate that readily binds platelets, microslides were coated with 3-aminopropyltriethoxysilane (APES; Sigma) as previously described.23 Blood was collected into sodium heparin (CP Pharmaceuticals Ltd, Wrexham, UK), and platelet-rich plasma (PRP) was removed after centrifuging the blood at 400g for 5 minutes. Platelets were counted, adjusted to 2 × 108/mL with PBS, loaded into microslides, and incubated at room temperature for 40 minutes to allow them to sediment, adhere, and form a confluent monolayer.21,22 Unbound platelets were removed by washing before assay. Experiments were always conducted using autologous platelets and neutrophils. Platelet monolayers prepared in this manner can capture and support the rolling adhesion of neutrophils for up to 20 minutes without evidence of platelet-mediated neutrophil activation.14
A 730 amino acid residue recombinant human P-selectin stop protein, lacking the transmembrane and cytoplasmic domains (affinity-purified from stably transfected CHO cells; gift of Ian Collins, R&D Systems, Abingdon, UK; also commercially available) was dissolved in PBS at a concentration of 5 μg/mL. The protein was immobilized by incubation in an APES-treated microslide for 60 minutes at 37°C. Free protein-binding sites were subsequently blocked with 1% BSA (fraction IV) for 60 minutes at 37°C. BSA is a sufficient ligand to support the β2-integrin–mediated adhesion and migration of neutrophils in our own experiments21 and in other experimental systems,24-26 including a study of adhesion-dependent degranulation in response to fMLP.18
Adhesion and elastase release by neutrophils under conditions of flow.
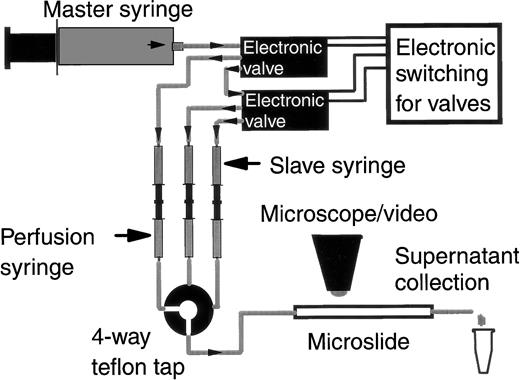
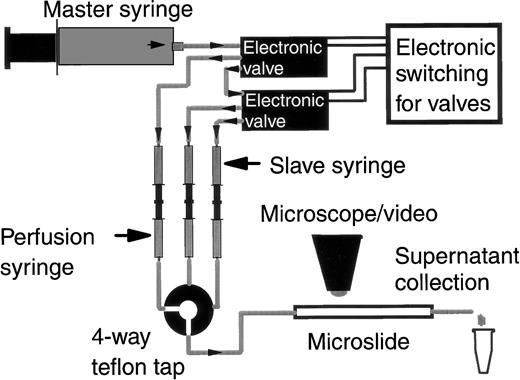
The assay system was a modification of that recently described14,27 and is shown schematically in Fig 1. A microslide containing adhesive substrate was attached to the common outlet of a four-port tap (Hamilton miniature valve; V.A. Howe Ltd, London, UK). Plastic, 2.5-mL perfusion syringes containing either chemotactic agent, PBS/BSA, or a suspension of neutrophils were connected to one of the three remaining tap inlets via flexible silicone tubing. These syringes were mounted back-to-back with identical water-filled slave syringes on a specially engineered rig (Fig 1) that held them immobile. Expulsion of the perfusate was driven at a controlled rate by a 50-mL master syringe on a Harvard syringe pump that pushed flow into the slave syringes. Selection between perfusates was achieved using two electronic valves (Lee Products Ltd, Gerards Cross, UK) that switched the route of the output from the master syringe to a chosen slave syringe. A constant wall shear stress of 0.05 Pa (or 0.5 dyn/cm2) was maintained in the microslide by choice of the appropriate flow rate. This is at the low end of the physiological range of wall shear stress in post capillary venules28 and was chosen so that large numbers of adherent neutrophils could be established and concentration of released substances maximized.
Diagrammatic representation of the flow-based adhesion assay incorporating an hydraulic-driven perfusion system allowing the collection of buffer perfused over adherent cells. The master syringe (driven by a Harvard syringe pump) expels water into one of three slave syringes. Switching between the slave syringes is via electronic valves. Each slave syringe is mounted back to back with and drives a perfusion syringe containing a different perfusate (wash buffer, cell suspension, or chemotactic agent). Output from the perfusion syringes is selected via a 4-way teflon tap, the common outlet of which is attached to a microslide containing the adhesive substrate. The microslide sits on the stage of a video-microscope. Perfused medium for analysis is collected from the distal end of the microslide into micro-centrifuge tubes.
Diagrammatic representation of the flow-based adhesion assay incorporating an hydraulic-driven perfusion system allowing the collection of buffer perfused over adherent cells. The master syringe (driven by a Harvard syringe pump) expels water into one of three slave syringes. Switching between the slave syringes is via electronic valves. Each slave syringe is mounted back to back with and drives a perfusion syringe containing a different perfusate (wash buffer, cell suspension, or chemotactic agent). Output from the perfusion syringes is selected via a 4-way teflon tap, the common outlet of which is attached to a microslide containing the adhesive substrate. The microslide sits on the stage of a video-microscope. Perfused medium for analysis is collected from the distal end of the microslide into micro-centrifuge tubes.
Microslides were mounted on the stage of a microscope fitted with a video camera, monitor, and recorder. The whole perfusion system was maintained at 37°C. Neutrophils were perfused across adhesive substrates at a concentration of 106/mL for 5 minutes. Perfused cells that were close to the wall of the microslide adhered via P-selectin and formed rolling attachments. The majority of neutrophils remained in the bulk flow of the perfusing buffer and were nonadherent. Nonadherent neutrophils were removed from the microslide with a 2-minute perfusion of wash buffer, leaving a large population (∼1,500/mm2) of continuously rolling, adherent neutrophils. A video record was made in at least five fields of view of known dimensions to allow the number of adherent cells to be counted. Continuously rolling neutrophils were then activated by the perfusion of PBS/BSA containing either fMLP (10−7 mol/L; Sigma), PAF (10−7 mol/L; Sigma), IL-8 (1 μg/mL; R&D Systems), or 1% zymosan (Sigma) -activated plasma (ZAP) as a source of C5a. ZAP was made by incubating 8 mg zymosan/mL of plasma for 30 minutes at 37°C, followed by centrifugation at 10,000g for 5 minutes and filtration through a sterile 0.2-μm filter. Upon receipt of a chemotactic stimulus, neutrophils rapidly stopped rolling and became stationary (∼1 to 2 seconds), changed shape and spread (∼0.5 to 1 minute), and began to migrate (1 minute onwards).14 21 Chemotactic agents were perfused continuously for 10 minutes and perfusate was collected from the outlet in 2.5-minute fractions for assay of elastase content. As controls, PBS/BSA alone was flowed over continuously rolling neutrophils and perfusate was collected in the same manner. Aliquots were centrifuged at 10,000g for 1 minute to remove detached cells and stored at −30°C until assay.
Adhesive requirements for neutrophil elastase release.
To investigate the adhesive requirements for elastase release, we blocked the neutrophil integrins CD11b and CD18 and the platelet receptor P-selectin. Antibodies to integrins were incubated with neutrophils for 15 minutes at room temperature. Because activated neutrophils rapidly mobilize granule stores of both CD18 and CD11b, it was also necessary to include MoAb at the same concentrations in the buffer containing fMLP to block newly expressed integrin molecules. Because pretreatment of platelet monolayers with antibody against P-selectin abolished all adhesion,22 we used a different strategy. We perfused the anti–P-selectin MoAb with the fMLP over a population of rolling neutrophils. This permitted neutrophil capture from flow and rolling adhesion before the delivery of fMLP but blocked P-selectin interactions after the initiation of β2-integrin–mediated adhesion. We have successfully used this strategy in a previous study to block P-selectin–mediated signals that modify the migration rate of activated neutrophils.21Perfusion of the antibody with fMLP does not affect the number of neutrophils that become stationary adherent.21
Enzyme-linked immunosorbent assay (ELISA) for neutrophil elastase.
Elastase concentrations in perfusates were determined using a commercial, colorimetric immunoassay (Merck Immunoassay PMN elastase; Merck, Lutterworth, UK) according to the manufacturer’s instructions. The assay measures the concentration of neutrophil elastase complexed with α1-antitrypsin. Because neither plasma nor serum was present in our assay, samples were first incubated for 30 minutes at 37°C with 100 μg/mL purified α1-antitrypsin (Sigma), which ensured a large molar excess over elastase. Reducing or increasing the α1-antitrypsin concentration by a factor of 10 did not influence the sensitivity of the assay (data not shown). The assay was calibrated against known concentrations of purified elastase-α1-antitrypsin complex provided with the kit and elastase concentration was normalized for the number of cells present in each microslide (nanograms per 106neutrophils). For comparison, neutrophils in suspension at known concentration were stimulated with the same agents for 10 minutes, and the supernatant was isolated after centrifugation at 10,000gfor 1 minute. The supernatant was assayed in the same manner as the perfusate. Total elastase content of neutrophils was also determined by lysing known numbers with 0.5% sodium dodecyl sulphate (SDS; Sigma) and isolation of the supernatant after centrifugation at 10,000g for 5 minutes.
Inhibition of 5-lipoxygenase and blockade of LTB4receptor.
In some experiments, neutrophils were treated with MK886 (a kind gift from Dr P. Vickers, MerckFrosst Centre for Therapeutic Research, Pointe Dorval, Canada), an inhibitor of 5-lipoxygenase activating protein (FLAP),29 at concentrations of 50 or 500 nmol/L for 10 minutes before experimentation. On other occasions, the LTB4 receptor antagonist LY223982 (Lilly Research Laboratories, Indianapolis, IN)30 was preincubated with neutrophils at a concentration of 100 μmol/L for 10 minutes. The agents were also included in all perfusates to which the neutrophils were exposed. Both reagents were solubilized in dimethyl sulfoxide (DMSO), and, after dilution, carrier concentrations were 0.1% or less. Control experiments using carrier alone showed no effect on degranulation in response to fMLP (data not shown).
Enzyme immunoassay for LTB4.
Perfusate from neutrophils exposed to fMLP or PAF in the presence or absence of MK886 were collected and stored at −70°C before analysis. LTB4 was quantified using a commercially available enzyme immunoassay (Amersham Life Science, Little Chalfont, UK) with a reported sensitivity of 6 pg/mL.
Statistical analysis.
Concurrent effects of time and different treatment group were tested using analysis of covariance, followed by one-way analysis of variance (ANOVA) for effect of time or dose on a single treatment where appropriate. Comparison of individual treatments or conditions at single time points was made using the paired t-test or unpaired (Student’s) t-test as appropriate. All tests were made using the computer program Minitab (Minitab Inc, State College, PA).
RESULTS
Elastase release from neutrophils adherent to platelet monolayers.
Neutrophils perfused over immobilized activated platelets at a wall shear stress of 0.05 Pa were captured from flow by P-selectin. The great majority of adherent cells were rolling (>85%) with a velocity of 1.9 ± 0.2 μm/sec (mean ± SEM of 60 cells from 3 experiments). The remaining cells spontaneously activated on the platelet monolayer and were stationary adherent. After superfusion of each chemotactic stimulus (fMLP, C5a, IL-8, or PAF), all neutrophils rapidly stopped rolling, spread, and migrated across the platelet monolayer. These responses have been described in detail elsewhere.14 21
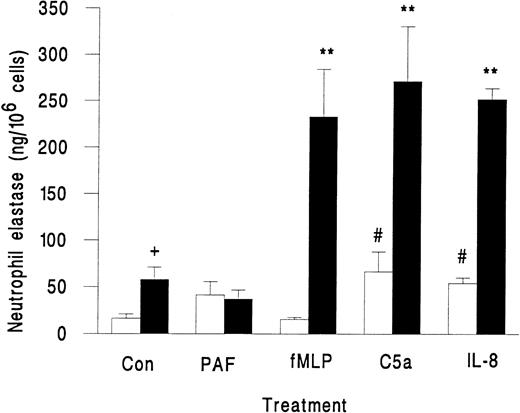
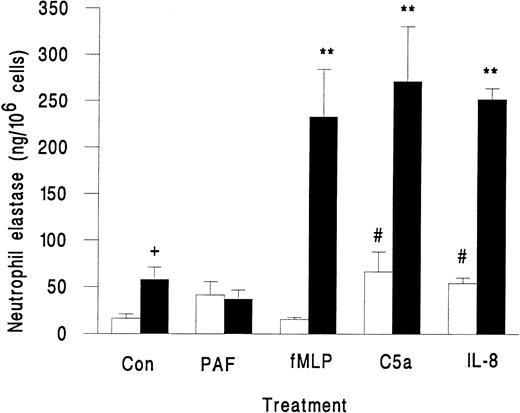
Neutrophils in suspension released little elastase when activated with fMLP, PAF, C5a, or IL-8, although the latter two agents did induce greater release than unstimulated control (Fig 2). However, when these agents were superfused over neutrophils that were rolling on platelets, there was a marked release of elastase, except in the case of PAF, which did not induce elastase release (Fig 2). We also observed a small but significant increase in elastase release when untreated neutrophils rolling on platelets were compared with those in suspension (Fig 2). This increase may represent release from those neutrophils that were spontaneously activated while in contact with the platelet monolayer. As a marker for activation, less than 2% of neutrophils in the original suspension showed pseudopod formation, but 13% ± 2% of neutrophils adherent to platelets were distorted in shape (mean ± SEM from 7 experiments). Total elastase content of neutrophils was 1.4 ± 0.2 μg/106 cells (mean ± SEM of 3 experiments). Elastase released by surface-adherent neutrophils activated with fMLP, C5a, or IL-8 over 10 minutes (Fig 2) equated to 20% to 25% of total neutrophil elastase.
Elastase release from unstimulated neutrophils (Con) or neutrophils treated with chemotactic agents (10−7 mol/L PAF, 10−7 mol/L fMLP, 1% ZAP as a source of C5a, or 1 μg/mL IL-8) either in suspension (□) or while adherent to platelet monolayers (▪). Data are the mean ± SEM from three to eight experiments. ANOVA showed significant effect of treatment and of adhesion on release of elastase. Paired t-test showed a significant increase (#P < .05) in elastase released from neutrophils in suspension activated by C5a and IL-8 compared with control; a significant increase (**P < .01) in elastase released from surface-adherent neutrophils activated by fMLP, C5a, or IL-8 compared with control; a significant increase (+P < .05) in elastase released from rolling unstimulated control neutrophils compared with unstimulated control neutrophils in suspension.
Elastase release from unstimulated neutrophils (Con) or neutrophils treated with chemotactic agents (10−7 mol/L PAF, 10−7 mol/L fMLP, 1% ZAP as a source of C5a, or 1 μg/mL IL-8) either in suspension (□) or while adherent to platelet monolayers (▪). Data are the mean ± SEM from three to eight experiments. ANOVA showed significant effect of treatment and of adhesion on release of elastase. Paired t-test showed a significant increase (#P < .05) in elastase released from neutrophils in suspension activated by C5a and IL-8 compared with control; a significant increase (**P < .01) in elastase released from surface-adherent neutrophils activated by fMLP, C5a, or IL-8 compared with control; a significant increase (+P < .05) in elastase released from rolling unstimulated control neutrophils compared with unstimulated control neutrophils in suspension.
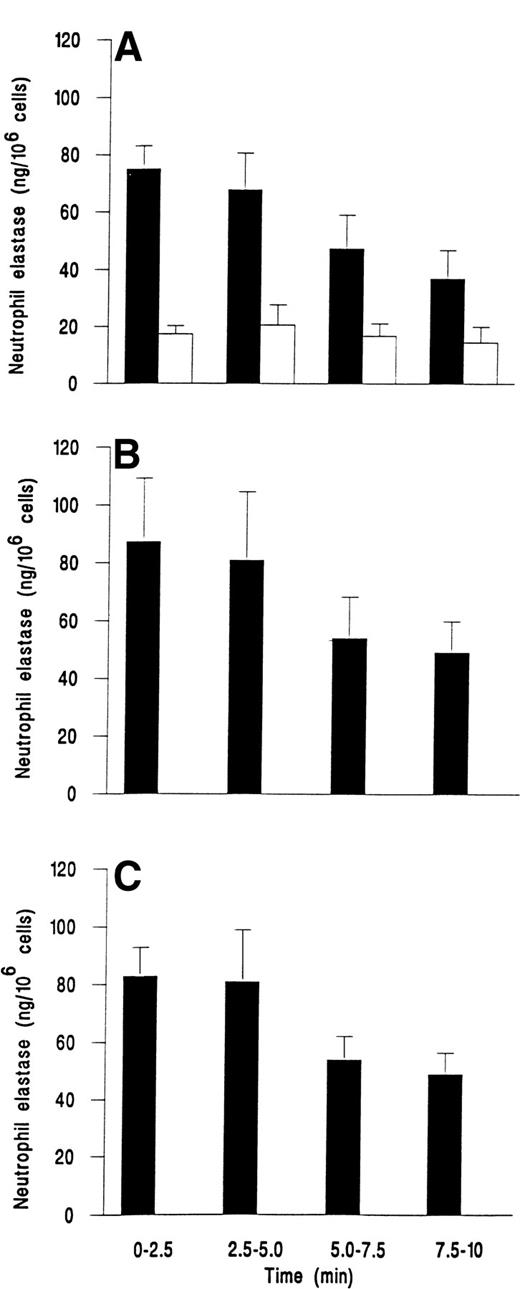
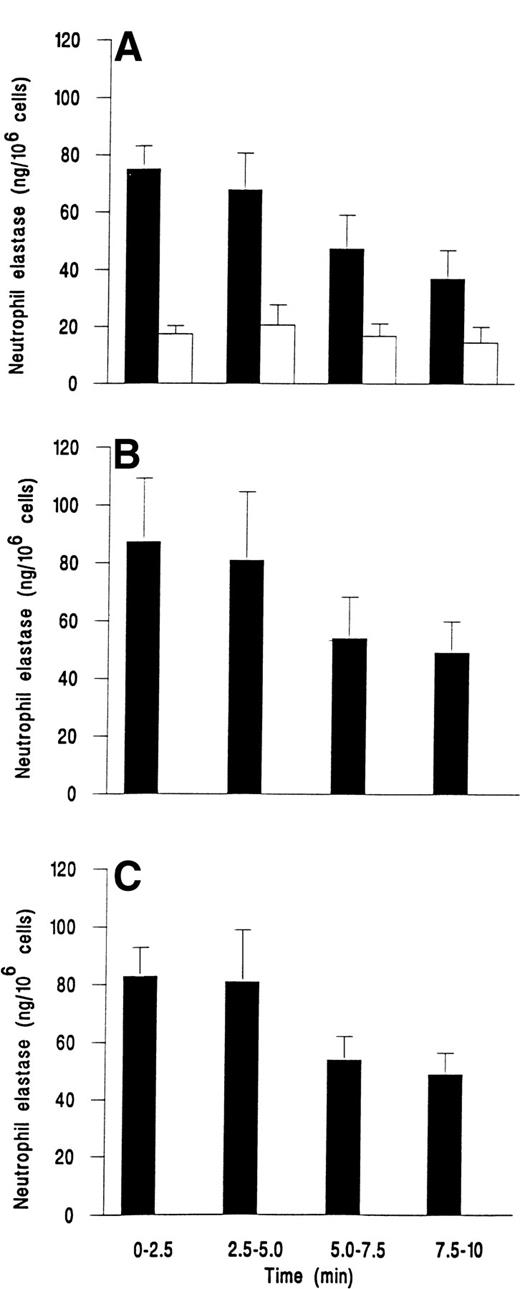
We followed the kinetics of elastase release by assaying perfusate collected at 2.5-minute intervals. Figure 3demonstrates that release was maximal within the first 2.5 minutes for fMLP, C5a, or IL-8. Elastase release then decreased slowly over the next 7.5 minutes, but was still greater than levels from unstimulated adherent cells that gave a steady low level of release over the 10 minutes (Fig 3).
Time course of elastase release from neutrophils adherent to platelets either (A) unstimulated and continuously rolling (□) or stimulated with 10−7 mol/L fMLP (▪); (B) stimulated with 1% ZAP as a source of C5a; or (C) stimulated with 1 ng/mL IL-8. Data are the mean ± SEM of least four experiments. Analysis of covariance showed that there was a significant effect of time and treatment on elastase release (P < .01) and that treatments with chemotactic agents were significantly different from control (P < .01), but not different from each other. One-way ANOVA showed that elastase release from control cells did not vary with time but that there was a significant effect of time on release for individual treatments with chemotactic agents (P < .05 in each case).
Time course of elastase release from neutrophils adherent to platelets either (A) unstimulated and continuously rolling (□) or stimulated with 10−7 mol/L fMLP (▪); (B) stimulated with 1% ZAP as a source of C5a; or (C) stimulated with 1 ng/mL IL-8. Data are the mean ± SEM of least four experiments. Analysis of covariance showed that there was a significant effect of time and treatment on elastase release (P < .01) and that treatments with chemotactic agents were significantly different from control (P < .01), but not different from each other. One-way ANOVA showed that elastase release from control cells did not vary with time but that there was a significant effect of time on release for individual treatments with chemotactic agents (P < .05 in each case).
Elastase release from neutrophils adherent to purified P-selectin and albumin.
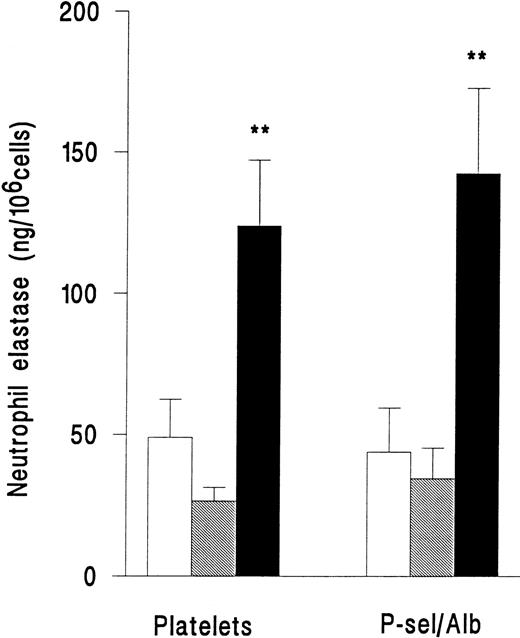
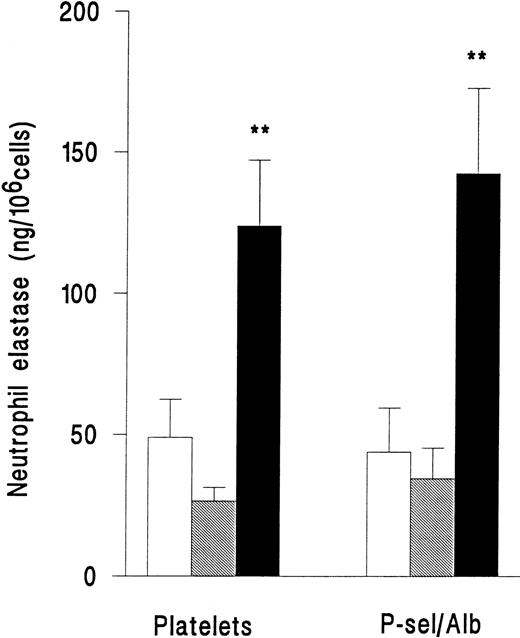
In a separate series of experiments, release of elastase from neutrophils adherent to platelets or adherent to purified P-selectin coimmobilized with albumin was compared. Superfusion of PAF did not induce an increase in release of elastase for either type of surface (Fig 4). However, fMLP induced release of elastase with equal efficiency on platelets or immobilized adhesion receptors (Fig 4). Adhesion per se was therefore required for neutrophil elastase release, but platelets were not essential.
Elastase release from neutrophils that were unstimulated and continuously rolling (□), stimulated with 10−7mol/L PAF (▧), or stimulated with 10−7 mol/L fMLP (▪) while adherent to platelet monolayers (Platelets) or 5 μg/mL purified recombinant P-selectin coimmobilized with 1% BSA (P-sel/Alb). Data are the mean ± SEM of three experiments. ANOVA showed that there was a significant effect of treatment on release of elastase but that adherent substrate did not affect release. Paired t-test showed that elastase release from fMLP-but not PAF-treated cells was significantly increased (**P < .01) compared with rolling unstimulated control cells on either adhesive substrate.
Elastase release from neutrophils that were unstimulated and continuously rolling (□), stimulated with 10−7mol/L PAF (▧), or stimulated with 10−7 mol/L fMLP (▪) while adherent to platelet monolayers (Platelets) or 5 μg/mL purified recombinant P-selectin coimmobilized with 1% BSA (P-sel/Alb). Data are the mean ± SEM of three experiments. ANOVA showed that there was a significant effect of treatment on release of elastase but that adherent substrate did not affect release. Paired t-test showed that elastase release from fMLP-but not PAF-treated cells was significantly increased (**P < .01) compared with rolling unstimulated control cells on either adhesive substrate.
Adhesive requirements for neutrophil elastase release.
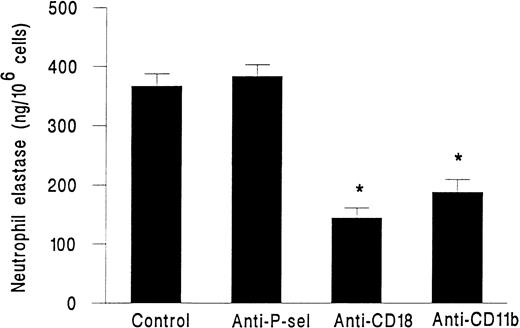
Using adhesion blocking MoAbs, we investigated the role of the neutrophil integrin CD11b/CD18 and the platelet rolling receptor P-selectin in elastase release from neutrophils activated with fMLP on platelet monolayers. In the presence of antibodies against CD11b or CD18 neutrophil, elastase release was significantly reduced by between 50% and 60%. However, blockade of P-selectin at the time of delivery of fMLP had no effect on elastase release (Fig 5).
Elastase release from neutrophils adherent to platelet monolayers in the presence of 10−7 mol/L fMLP (Control) and an MoAb against either P-selectin (Anti-P-sel), CD18 (Anti-CD18), or CD11b (AntiCD11b). Data are the mean ± SEM from three experiments. Paired t-test showed that elastase release from cells in the presence of CD18 or CD11b antibodies was significantly lower (*P < .05) than in untreated control cells.
Elastase release from neutrophils adherent to platelet monolayers in the presence of 10−7 mol/L fMLP (Control) and an MoAb against either P-selectin (Anti-P-sel), CD18 (Anti-CD18), or CD11b (AntiCD11b). Data are the mean ± SEM from three experiments. Paired t-test showed that elastase release from cells in the presence of CD18 or CD11b antibodies was significantly lower (*P < .05) than in untreated control cells.
Role of leukotrienes in elastase release.
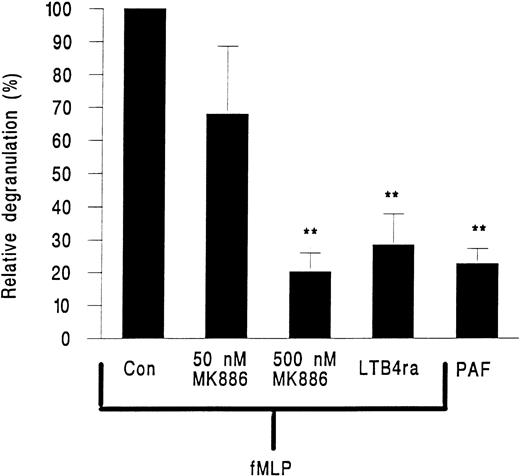
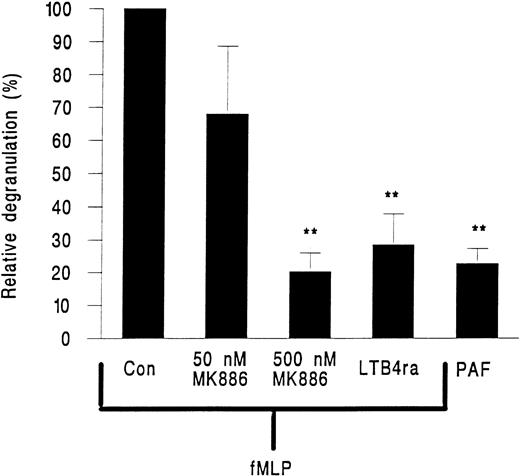
MK886, a FLAP inhibitor and hence an inhibitor of leukotriene generation, and the LTB4 receptor antagonist LY223982 were tested for their effects on neutrophil elastase release. MK886 at 50 nmol/L inhibited approximately 40% of elastase release and at 500 nmol/L elastase release was reduced by about 80% (Fig 6). LY223982 used at 100 μmol/L also essentially abolished the release of elastase from neutrophils in response to fMLP (Fig 6).
Elastase release from neutrophils adherent to platelet monolayers in the presence of 10−7 mol/L PAF or 10−7 mol/L fMLP and either 50 or 500 nmol/L of the FLAP inhibitor MK886 or 100 nmol/L of the LTB4 receptor antagonist LY223982 (LTB4 ra). Data are expressed relative to values obtained from paired samples stimulated with fMLP alone (Con). Data are the mean ± SEM of six experiments using PAF or LY223982 and of three experiments for each intervention with MK886. Paired t-test showed that elastase release was significantly lower (**P < .01) than fMLP control for cells treated with PAF or cells treated with fMLP and LY223982 or 500 nmol/L MK886.
Elastase release from neutrophils adherent to platelet monolayers in the presence of 10−7 mol/L PAF or 10−7 mol/L fMLP and either 50 or 500 nmol/L of the FLAP inhibitor MK886 or 100 nmol/L of the LTB4 receptor antagonist LY223982 (LTB4 ra). Data are expressed relative to values obtained from paired samples stimulated with fMLP alone (Con). Data are the mean ± SEM of six experiments using PAF or LY223982 and of three experiments for each intervention with MK886. Paired t-test showed that elastase release was significantly lower (**P < .01) than fMLP control for cells treated with PAF or cells treated with fMLP and LY223982 or 500 nmol/L MK886.
We also analyzed neutrophil production of LTB4 by enzyme immunoassay (EIA). Neutrophils in suspension released no detectable LTB4 with or without the addition of PAF (3 experiments; signal below detection limit of EIA), but, when stimulated by fMLP production of LTB4, was detected, but at low concentrations (23.5 ± 3.1 pg/106 cells; mean value ± SEM, n = 5). When surface adherent neutrophils were activated with fMLP, LTB4 was detectable in perfusate (41.7 ± 6.8 pg/106 cells; mean value ± SEM, n = 5). When PAF was used to activate surface adherent neutrophils in three paired experiments, LTB4 was undetectable in the perfusate in two of the three experiments. Pretreatment of surface adherent neutrophils with the FLAP inhibitor, MK886, followed by challenge with fMLP resulted in a reduction in LTB4 generation to levels less than the detection limit of the assay. Finally, we established that LTB4 could induce elastase release. In two experiments, neutrophils adherent to platelet monolayers and stimulated with 10−7 mol/L LTB4 generated 168 ± 14 ng of elastase/106 cells, whereas unstimulated control neutrophils released only 63 ± 2 ng of elastase/106cells.
DISCUSSION
For the first time we have investigated the control of elastase release from the primary granules of neutrophils in a flow-based model of the circulation. When neutrophils were perfused with fMLP, C5a, or IL-8 while rolling on P-selectin presented by a platelet monolayer, they became immobilized and showed marked release of elastase. Interestingly, although PAF also induced activation and immobilization of rolling neutrophils, it was an insufficient stimulus to promote elastase release. When compared with freely suspended cells, elastase release was amplified greatly by adhesion via CD11b/CD18 to a substrate of platelets or purified albumin. Release was comparable on platelet- or protein-coated surfaces, so that agents derived from platelets, such as eicosanoids or platelet activating factor,31 were not essential requirements. Elastase release was maximal within 2.5 minutes after chemotactic stimulation, but significant release was maintained for at least 10 minutes and totalled approximately 25% of elastase stores. Neutrophils activated with fMLP released low levels of LTB4. An inhibitor of 5-lipoxygenase–activating protein (MK 886) and an LTB4 receptor antagonist (LY223982) each reduced the release of elastase markedly. Thus, eicosanoids and, specifically, LTB4 generated via the 5-lipoxygenase pathway in neutrophils acted in an autocrine manner to promote elastase release.
These studies were made possible by the development of a novel perfusion system. Hydraulically driven syringes allowed sequential perfusion of neutrophils, wash buffer, and chemotactic agents over an adhesive substrate and timed collection of perfusate for analysis of released compounds. This approach has several advantages over assays of neutrophil degranulation using suspensions. It more closely models the in vivo situation, in which degranulation occurs mostly in adherent cells, and obviates the need to artificially prime the neutrophils with cytochalasin B, an essential step for the release of primary granules in suspension.1,15,16 We used an adhesive surface of activated platelets (that might resemble the situation in thrombotic vessels) or purified P-selectin and albumin, but other substrates might be chosen to allow receptor-specific modulation of degranulation to be studied. Flow-based adhesion assays are well adapted to follow rapid changes in adhesive behavior of activated neutrophils14 21and here allowed the secretory and/or synthetic responses of neutrophils to be monitored continually. Currently, perfusate was collected at intervals of 2.5 minutes (perfused volume, ∼0.5 mL) because of initial uncertainty regarding the concentration of released substances. In fact, collected samples required dilution 10-fold for elastase assay and were sufficient for repeated assays of this or other compounds. Thus, for a plentifully released compound such as elastase, greater resolution of the kinetics of release could have been achieved by collecting smaller samples of the perfusuate more frequently.
Elastase release occurred with equal efficiency on immobilized activated platelets or on purified recombinant P-selectin coimmobilized with albumin. This implied that the adhesion-dependent signal acted through a neutrophil P-selectin ligand and/or the neutrophil β2-integrin CD11b/CD18 that supports activation-dependent adhesion to platelets14,21,32 or immobilized albumin.21 When we used MoAbs to block neutrophil adhesion to platelets, we found that anti-CD18 or anti-CD11b but not anti–P-selectin blocked elastase release. This agrees closely with data from a static adhesion system in which primary granule release from fMLP-stimulated neutrophils adherent to albumin was blocked by MoAb against CD18 or CD11b.18 Interestingly, increased expression of CD11b/CD18 on neutrophils, which is indicative of neutrophil activation, correlated with elastase release during extracorporeal circulation of blood,19 whereas antibody-blockade of CD18 inhibited neutrophil elastase release induced by exposure of blood to hemodialysis membranes.20CD11b/CD18 binding to albumin was also required for the degranulation of another member of the granulocyte lineage, the eosinophil, in response to granulocyte-macrophage colony-stimulating factor or PAF.33 Thus, there is now compelling evidence to suggest that the release of neutrophil elastase from primary granules is an activation-driven process that requires adhesion via the glycoprotein CD11/CD18.
Activation-dependent adhesion via β2-integrins was essential for the efficient release of elastase from neutrophils. Release of elastase from rolling adherent unactivated neutrophils or from neutrophils activated in suspension was minimal. However, whereas activation-dependent adhesion was essential for primary granule release, it was not necessarily sufficient, because PAF was unable to induce release of elastase. The inability of PAF to induce neutrophil elastase release was not due to the modulatory effects of agents generated by activated platelets. Although activation of flowing neutrophils by platelet-derived stimuli such as LTB4 and PAF has been previously reported,31 we could demonstrate no qualitative or quantitative differences in neutrophil responses to stimulation with fMLP or PAF when experiments were conducted on platelets or on a substrate of purified adhesion receptors. Thus, any platelet-derived agents did not modify the response to fMLP or PAF. However, the low levels of stationary adhesion and elastase release observed here on platelet monolayers without exogenous stimulation suggest the presence of platelet-derived stimuli, although presumably PAF did not cause release of elastase.
The route by which adhesive and chemotactic signals are integrated to control degranulation remains uncertain. It is possible that elastase release is influenced by changes in the cytoskeleton of the cell dependent on adhesion and not linked to signaling from an integrin receptor. Activation-dependent adhesion is generally associated with dynamic rearrangement of the actin cytoskeleton.13Moreover, cytoskeletal disruption by cytochalasin B allows neutrophil primary granule release in suspension,1,15,16 although the release of other granule compartments may occur via a cytochalasin-insensitive route.17 Cytochalasin B has been suggested to remove a mechanical barrier to granule translocation to the outer membrane by the degradation of F-actin (for review, see Smolen1). Thus, cytoskeletal remodelling may provide a common mechanism allowing primary granule mobilization in both surface-adherent and cytochalasin-primed neutrophils. An alternative route by which cytochalasin B could enhance degranulation is via increased production of diacylglycerol leading to protein kinase C-dependent neutrophil priming.34 35 Increased mobilization of this second messenger in cytochalsin B-treated and surface-adherent neutrophils could also provide a common route to primary granule release.
Studies with the FLAP-inhibitor, MK 886, and LTB4 receptor antagonist LY223982 and measurements of released LTB4strongly indicated that LT synthesis was required for elastase release. The levels of LTB4 in perfusate were near the limit of detection by EIA and were equivalent to about 5 to 10 pg/mL. Previous studies have shown that, in neutrophils stimulated with C5a, LTB4 can be retained within the cell and operate as an intracellular second messenger rather than a secreted chemotactic agent.36 This may account for the evident response at the low levels of this eicosanoid found in perfusates. Moreover, autocrine stimulation of degranulation by newly synthesized LTB4 has previously been demonstrated in human neutrophils activated by the Ca2+ ionophore A23187.37 There are several other reports that LTB4 can cause degranulation in mammalian neutrophils,38,39 and this response is augmented in the presence of other lipid mediators such as PAF and 5(S)-hydroxyeicosatetraenoic acid.39 We further established that the addition of 10−7 mol/L LTB4 induced elastase release in the current model. Interestingly, neutrophils from patients with rheumatoid arthritis treated with Tenidap sodium (a 5-lipoxygenase inhibitor) demonstrated a reduction in ability both to synthesize LTB4 and to release neutrophil elastase.40 The above-mentioned results indicate that the inability of PAF to promote degranulation and to induce the synthesis of LTB4 may be linked. The differential ability of PAF and fMLP to promote LTB4 synthesis may arise because the receptors for these agents can be coupled to different G proteins and thus may signal via separate second messenger cascades.41-44 Nevertheless, PAF has been shown to cause elastase release from neutrophils in the presence of cytochalasin B39,45 and has been shown to prime neutrophils adherent to endothelium for enhanced elastase release in response to a secondary challenge with fMLP.46
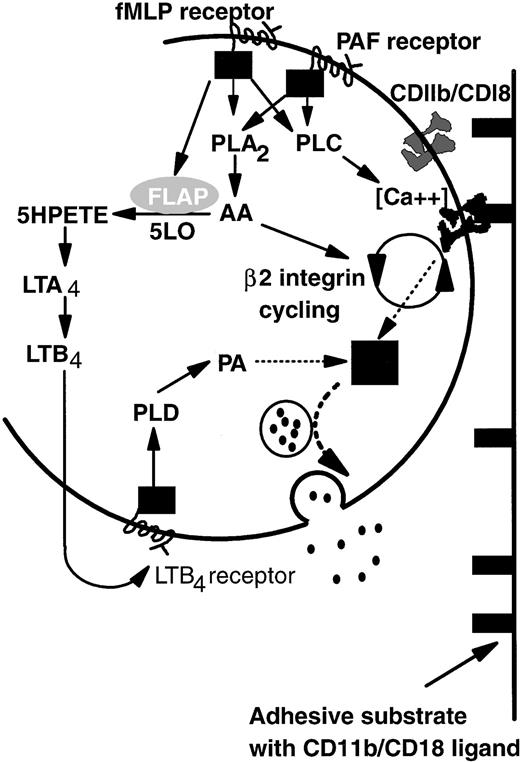
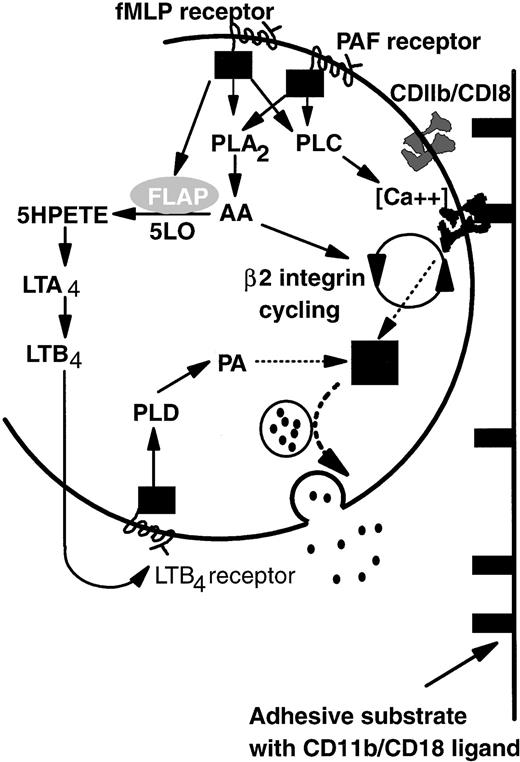
Based on the above-noted considerations and previous studies of intracellular signaling pathways, we propose a model that explains common adhesive, but divergent degranulation responses induced by PAF and fMLP (Fig 7). On binding of PAF or fMLP, specific G-protein–linked receptors activate both phospholipase A2 (PLA2) and phospholipase C (PLC).41,47,48 Activation of PLC then upregulates β2-integrin function,49-51 possibly by controlling local Ca2+ concentration.52PLA2 causes release of arachidonic acid (AA) that is essential for de novo expression and activation (cycling) of β2-integrin CD11b/CD18 from the tertiary granule stores48,53 and thus is important in prolonged neutrophil adhesion.14,53 Continual binding of CD11b/CD18 acts as a signal which promotes primary granule release but is itself insufficient. An additional pathway arises through activation of 5-lipoxygenase and, thus, LTB4 synthesis induced by fMLP but not PAF. LTB4 generated intracellularly or possibly also via a transcellular route (pathway not shown; for review, see Serhan54) binds in turn to a specific G-protein–linked receptor and promotes the production of phosphatidic acid (PA) by the action of phospholipase D (PLD) on membrane phospholipids.55 PA has been reported to be essential for neutrophil primary granule release55 and acts as the second signal integrated with that from integrin-binding to induce elastase secretion. We have represented the uncertainty surrounding integration of these two signals as a black box. Ultimately granules containing elastase are translocated to and fuse with the external lipid membrane, liberating their contents into the extracellular environment.
A model of the differential control of neutrophil degranulation by the chemotactic agents fMLP and PAF. Both fMLP and PAF induce PLA2 and PLC activation via G-protein–linked serpentine receptors. PLC may regulate β2-integrin–mediated adhesion by modulating local calcium fluxes. AA may be required for CD11b/CD18 cycling (de novo expression and activation), a process necessary for prolonged neutrophil adhesion and migration. Signaling via the fMLP receptor but not the PAF receptor also mobilizes FLAP, which in turn activates five lipoxygenase (5LO). LTB4 is synthesized by the action of 5LO on arachidonic acid. Details are not shown, but the intermediate metabolite LTA4 may also be transformed by intercellular pathways. The binding of LTB4 to a specific G-protein–linked serpentine receptor activates PLD, the action of which generates PA from membrane phospholipids. Signals from PA and ligand-bound CD11b/CD18 are integrated in an unknown manner (▪) that results in granule mobilization, fusion with the outer cell membrane, and release of contents into the extracellular environment (see text for abbreviations).
A model of the differential control of neutrophil degranulation by the chemotactic agents fMLP and PAF. Both fMLP and PAF induce PLA2 and PLC activation via G-protein–linked serpentine receptors. PLC may regulate β2-integrin–mediated adhesion by modulating local calcium fluxes. AA may be required for CD11b/CD18 cycling (de novo expression and activation), a process necessary for prolonged neutrophil adhesion and migration. Signaling via the fMLP receptor but not the PAF receptor also mobilizes FLAP, which in turn activates five lipoxygenase (5LO). LTB4 is synthesized by the action of 5LO on arachidonic acid. Details are not shown, but the intermediate metabolite LTA4 may also be transformed by intercellular pathways. The binding of LTB4 to a specific G-protein–linked serpentine receptor activates PLD, the action of which generates PA from membrane phospholipids. Signals from PA and ligand-bound CD11b/CD18 are integrated in an unknown manner (▪) that results in granule mobilization, fusion with the outer cell membrane, and release of contents into the extracellular environment (see text for abbreviations).
Differential, adhesion-dependent response of neutrophils to chemotactic agents may be physiologically important. The release of primary granule contents is not appropriate for neutrophils suspended in blood or for adherent cells in the process of migrating from the vessel lumen. PAF is a neutrophil-activating agent ordinarily found on cytokine-stimulated endothelium, which promotes immobilization of neutrophils and their transendothelial migration.3,56 This processes requires the rapid mobilization of CD11b/CD181,12,13 from tertiary granules,1 but does not require potentially harmful primary granule release. Therefore, PAF may promote neutrophil adhesion and migration across the endothelial barrier of blood vessels without inducing primary granule release. In contrast, activating agents ordinarily restricted to the extravascular tissues during inflammation, for instance, bacterial peptides and activated complement fragments, can induce primary granule release as well as tertiary granule mobilization. Such responses would be most suited to phagocytosis and destruction of invasive micro-organisms in the extravascular tissues during inflammation. Thus, we advance a paradigm in which transendothelial migration of neutrophils occurs in response to agents such as PAF, while ensuring that defense mechanisms such as the mobilization of primary granules and the release of their contents is only triggered in the tissues by chemotactic agents concentrated there. Disruption of this model, for instance, by delivery of inappropriate activating agents to the vascular compartment, could be the basis of pathological degranulation of adherent neutrophils, whereas interventions directed against the pathway acting through 5-lipoxygenase might be protective against tissue damage.
ACKNOWLEDGMENT
The authors are grateful to Prof Charles Serhan for helpful comments regarding the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to G. Ed Rainger, PhD, Department of Physiology, The Medical School, The University of Birmingham, Birmingham B15 2TT, UK; e-mail: raingege@bham.ac.uk.