Abstract
Glycophorin A is the major transmembrane sialoglycoprotein of red blood cells. It has been shown to contribute to the expression of the MN and Wright blood group antigens, to act as a receptor for the malaria parasite Plasmodium falciparum and Sendai virus, and along with the anion transporter, band 3, may contribute to the mechanical properties of the red blood cell membrane. Several lines of evidence suggest a close interaction between glycophorin A and band 3 during their biosynthesis. Recently, we have generated mice where the band 3 expression was completely eliminated by selective inactivation of the AE1 anion exchanger gene, thus allowing us to study the effect of band 3 on the expression of red blood cell membrane proteins. In this report, we show that the band 3 −/− red blood cells contain protein 4.1, adducin, dematin, p55, and glycophorin C. In contrast, the band 3 −/− red blood cells are completely devoid of glycophorin A (GPA), as assessed by Western blot and immunocytochemistry techniques, whereas the polymerase chain reaction (PCR) confirmed the presence of GPA mRNA. Pulse-label and pulse-chase experiments show that GPA is not incorporated in the membrane and is rapidly degraded in the cytoplasm. Based on these findings and other published evidence, we propose that band 3 plays a chaperone-like role, which is necessary for the recruitment of GPA to the red blood cell plasma membrane.
AMONG THE TRANSMEMBRANE sialoglycoproteins of the red blood cell, glycophorin A (GPA) was the first protein to be sequenced and has been the focus of extensive investigations in recent years (for reviews see Chasis and Mohandas1 and Fukuda2). This 36-kD protein represents the major sialoglycoprotein of the red blood cell membrane displaying about one million copies per cell. Its orientation places its N-terminal domain outside the cell, with a single transmembrane domain, thus connected to the cell interior via its C-terminal cytoplasmic domain.3,4 Despite its extensive characterization, the functional significance of GPA remains poorly understood. Although GPA contributes to the expression of blood group antigens, may modulate red blood cell membrane mechanical properties,1,2 and serves as an attachment site for the malaria parasite Plasmodium falciparum5,6 and Sendai virus,7 its complete loss in human red blood cells is not associated with any detectable alterations in shape, function, or lifespan.8,9 Similarly, red blood cells with genetic variants of GPA have been described and appear to exhibit normal physiologic properties.10 11
To investigate the functional roles of GPA, previous studies have focused on the putative interaction of GPA with the band 3 anion transporter of the red blood cell membrane. Band 3, which is present in a stoichiometrically comparable amount (≈ 1.2 × 106 copies per red blood cell), consists of two structural domains as defined by limited digestion with selective proteases (for review, see Tanner12 and Low13). The N-terminal 43-kD segment constitutes the cytoplasmic domain, which interacts with several proteins including glycolytic enzymes,14-16hemichromes,17-19 protein 4.2,12,13 and ankyrin.20-23 The interaction of band 3 with ankyrin provides a mechanism for coupling the membrane skeleton to the lipid bilayer. In contrast, the C-terminal 52 kD domain of band 3 forms multiple membrane-spanning segments through the lipid bilayer and facilitates the efflux of HCO3− from the red blood cell in exchange for Cl− (for review, see Jennings24).
Although a direct interaction between GPA and band 3 has not yet been documented, several previous studies suggest that the two proteins do interact in vivo. Evidence supporting this claim was initially provided by the observation that the glycosylation of band 3 is altered in GPA deficient En(a−) red blood cells, as well as in other GPA mutant red blood cells.25-27 These results suggested that GPA directly or indirectly modulates the posttranslational modification of band 3 in vivo. In addition, anti-GPA antibodies reduce the rotational diffusion of band 3 suggesting that the two proteins may associate in the red blood cell membrane.28 This view is further strengthened by the observation that the binding of anti-GPA antibodies to its extracellular domain rigidifies the red blood cell membrane29 and leads to the immobilization of both GPA and band 3 as measured by in situ FRAP (fluorescence recovery after photobleaching) technique.30 Furthermore, reconstitution of purified GPA and band 3 in nonionic detergents indicates that GPA may directly associate with band 3, albeit weakly.31 Recently, GPA was shown to facilitate the expression of band 3 in Xenopusoocytes and enhance its anion transport function.32 In addition, immunologic studies also support the view of a close GPA-band 3 interaction: a monoclonal antibody raised against band 3 was shown to coprecipitate GPA from red blood cell membranes.33-35Finally, the expression of the antithetical antigens Wraand Wrb, which represent alternative polymorphisms of band 3, requires an interaction between band 3 and GPA involving residues of the transmembrane domain of GPA.36 The presence of additional blood group epitopes, which are dependent on the interaction of GPA with band 3, was also suggested,37,38 and the existence of one such epitope was recently documented.39Taken together, these observations strongly suggest that GPA and band 3 interact at the red blood cell membrane.
Using gene targeting techniques, we and others have recently generated mice lacking the band 3 (AE1) protein in red blood cells.40,41 The development of band 3 −/− mice provides a unique opportunity to study the molecular basis of band 3-GPA interaction in vivo. Here we show that the membrane of mature erythrocytes obtained from band 3 −/− mice is completely devoid of GPA, as assessed by Western blot and immunofluorescence techniques. Furthermore, consistent with the presence of GPA mRNA, the GPA protein is synthesized in band 3 −/− erythroblasts, but fails to assemble on the plasma membrane. Based on these observations, and other published studies,32-39 we propose that a complex between band 3 and GPA is formed before the delivery of these proteins to the plasma membrane and that the band 3 protein plays an essential role in the recruitment of GPA to the red blood cell membrane.
MATERIALS AND METHODS
Reagents.
All reagents for electrophoresis and Western blot analysis were purchased from Bio-Rad Laboratories (Richmond, CA). Enzyme-coupled antibodies were purchased from Zymed, Inc, San Francisco, CA. The enhanced chemiluminescence (ECL) kit, purchased from Amersham, Arlington Heights, IL, was used for Western blot analysis.
Western blotting.
Peripheral blood was collected in EDTA by tail bleeding of normal and band 3 −/− adult littermates. The cells were washed three times in phosphate-buffered saline (PBS) (5.0 mmol/L Na2HPO4, pH 8.0, 0.5 mmol/L EGTA, 150 mmol/L NaCl) at 4°C. Washed red blood cells were lysed in 10 volumes of ice cold lysis buffer (5.0 mmol/L Na2HPO4, pH 8.0, 0.5 mmol/L EGTA, 2.0 mmol/L phenylmethyl sulfonyl fluoride [PMSF]). Ghosts were prepared by repeated washing of the pelleted membranes in lysis buffer at 4°C. The ghosts were then immediately solubilized by boiling in Laemmli sodium dodecyl sulfate (SDS) sample buffer and processed for electrophoresis (10% acrylamide gels) and Western blot analysis using the ECL kit, as described by the manufacturer. Antibodies against protein 4.2 were obtained from Catherine Korsgren of our department, and glycophorin C from Dr Philip Low of Purdue University (West Lafayette, IN). Details of the antibodies against band 3, dematin, and p55 have been published before.40,42 43 Rabbit antibodies against adducin were raised against purified human erythrocyte adducin (our unpublished data). Polyclonal serum recognizes both α and β subunits of adducin in erythrocyte ghosts.
Immunocytochemistry of red blood cells.
Blood was collected in Heparin after cardiac puncture from normal and band 3 −/− adult mice. The samples were centrifuged at 8,000 rpm onto Alcian Blue-coated coverslips using cytospin 3 (Shandon, Philadelphia, PA). The cells were then fixed for 15 minutes using a 4% paraformaldehyde solution, pH 7.4 (Baxter, Deerfield, IL). This was followed by three washes in PBS. The red blood cells were then permeabilized by incubation for 5 minutes in a PBS solution containing 0.1% Triton X-100 (Sigma, St Louis, MO). After washing with PBS, the cells were blocked overnight by incubation in a PBS solution containing 1.0% bovine serum albumin and 1.0% normal goat serum at 4°C. The coverslips were washed again at room temperature, and incubated with 200 μL of PBS solution containing the glycophorin A polyclonal antibody. These rabbit antibodies were raised against the cytoplasmic domain of human GPA (a gift from Dr M. Fukuda, La Jolla Cancer Research Foundation, La Jolla, CA). The coverslips were incubated overnight at 4°C with serum diluted to 1:50, washed three times with PBS, and the signal was detected with the goat antirabbit/rhodamine-conjugated secondary antibody (1:100 dilution in PBS). As controls, both normal and band 3 −/− red blood cells were treated with the secondary antibody alone. The coverslips were mounted onto slides using Fluoro Guard AntiFade reagent (Bio-Rad Laboratories, Melville, NY). Photographs were taken on the Nikon confocal microscope under 60X oil immersion lens.
Isolation of RNA and reverse transcriptase-polymerase chain reaction (RT-PCR).
RNA was prepared from the spleens of normal and band 3 null littermates. Spleens were removed and minced in a small volume of cold PBS on ice. Total RNA was then isolated using the Ultraspec isolation system (Biotecx Laboratories, Houston, TX). cDNA was synthesized from 1.0 μg of total RNA using the Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega Corp, Madison, WI), with random hexamer oligonucleotide primers under standard reaction conditions.44 An aliquot of each cDNA was then amplified by PCR using primers specific for mouse GPA45(sense primer 5′-CCCAGTATGACCGAGAGCACA-3′ and antisense primer 5′-TCTTCATTAGGAGTCTGCTCA-3′), which yielded a product of 497 bp. An aliquot of each cDNA was also used to amplify band 3 (sense primer 5′-CTCAGCCAGTCACAGAG-3′ and antisense primer 5′-GCTCCACATAGACCTGACC-3′) and β globin (sense primer 5-TGGTGCACCTGACTGATG-3′ and antisense primer 5′-GTGGTACTTGTGAGCCAA-3′) specific sequences. The expected PCR products using the band 3 and the β globin primers are 362 bp and 420 bp in length, respectively. An aliquot of each PCR reaction was analyzed by electrophoresis on a 1.5 % (wt/vol) agarose gel.
Biosynthesis of GPA in erythroblasts.
Band 3 −/− spleens were placed in ice cold Iscove's modified Dulbecco's medium (IMDM, GIBCO Laboratories, Grand Island, NY). A single cell suspension of spleen was obtained by disrupting the tissue with tweezers and passing through a polyethylene mesh (Spectramesh, Spectrum Medical Industries, Los Angeles, CA) of pore size 202 μm. The cell suspension was subjected to a discontinuous Percoll gradient (Percoll; Pharmacia Fine Chemicals, Piscataway, NJ) consisting of 45%, 65%, 70%, 77%, and 90% Percoll in IMDM.46 Fraction 4 consisted almost entirely of late (polychromatophilic and orthochromatic) erythroblasts as judged by staining with Wright-Giemsa and benzidine-hematoxylin. Morphologically identical control erythroblasts were similarly obtained from spleens of Balb/c mice 25 days after infection with Friend anemia virus (FVA).47 Control and B3−/− erythroblasts were metabolically labeled with [35S]methionine (30 μCi/mL, 1,000 Ci/mmol, ICN Biomedicals, Irvine, CA) for 60 minutes and chased with unlabeled methionine (0.4 mmol/L) for different time periods.
[35S]methionine-labeled cells were lysed in hypotonic buffer (10 mmol/L Tris-HCl, pH 7.5, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L PMSF, 1 mmol/L leupeptin, and 10 μg/mL aprotinin) and then disrupted with 10 strokes of a tight-fitting Dounce homogenizer. An appropriate volume of 2.0 mol/L sucrose was added immediately to restore isotonicity. The homogenate was centrifuged at 800g for 5 minutes to remove nuclei. The resulting supernatant was centrifuged at 18,000g for 20 minutes to separate the insoluble plasma membrane fraction from the soluble fraction containing fragments of endoplasmic reticulum (ER). The latter could be purified by centrifugation of the soluble fraction at 100,000g for 2 hours followed by fractionation on a concanavalin A column, but in the present study, this purification step was omitted because of the limited quantity of erythroblasts. GPA was immunoprecipitated from both the soluble and membrane fractions using rabbit polyclonal antibodies against the cytoplasmic domain of GPA.
RESULTS AND DISCUSSION
A large body of evidence supports the notion that band 3 may interact with GPA in the red blood cell membrane.12,32-39 However, a limitation to the in vivo documentation of such an interaction is the lack of vertebrate models displaying homozygous loss of band 3 in human red blood cells. The recent development of band 3 −/− animal models40,41,48 allowed us to investigate the consequences of band 3 deficiency on the assembly of membrane proteins in murine red blood cells. We first investigated the presence of GPA on the red blood cell membranes of band 3 −/− mice by Western blot analysis. Polyclonal antibodies raised against the cytoplasmic domain of human GPA were used to detect the level of GPA in the murine red blood cell membranes. It should be noted that the cytoplasmic domains of human and mouse GPA share 46% sequence identity (Lasergene MEGALIGN program, Irvine, CA), and as a consequence, the predicted antigenic indexes of the cytoplasmic domains of human and murine GPA are remarkably similar suggesting conservation of the immunoreactive epitopes in the two species (data not shown). Therefore, antibodies raised against human GPA would be expected to cross-react with murine GPA. Using these antibodies, we examined red blood cell membranes of band 3 −/− mice and their wild-type littermates for the presence of GPA. The samples analyzed on polyacrylamide gels were purposely overloaded to detect trace amounts of GPA. As shown in Fig 1, Western blotting failed to detect any measurable amount of GPA in the red blood cell membrane of band 3 −/− mice. In contrast, the dimeric form of GPA was detected in the red blood cell membranes of wild-type littermates (Fig 1). The absence of GPA in band 3 −/− red blood cells was confirmed in three additional mice of different ages ranging from 1 to 4 months (data not shown). It is relevant to mention here that the anti-GPA antibodies used in this study would not detect the presence of glycophorin B (GPB) and glycophorin E (GPE), as these proteins lack the cytoplasmic domain of GPA.1 2 Therefore, the status of GPB and GPE in band 3 −/− red blood cells remains to be investigated.
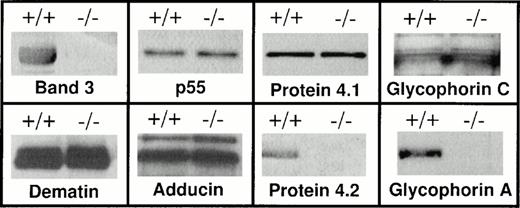
Western blot analysis of the red blood cell membrane proteins. Red blood cells were collected from both band 3 +/+ and band 3 −/−mice and washed in PBS. Ghosts were prepared by lysis as described in Materials and Methods. Ghosts membranes were solubilized and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to nitrocellulose, Western blot analysis was performed using the ECL chemiluminescence kit as suggested by the manufacturer. Both band 3 +/+ and band 3 −/− red blood cell membranes contain protein 4.1, glycophorin C, p55, dematin, and adducin, while the complete absence of protein 4.2 and GPA is noted. The GPA panel shows the position of the dimer. No monomeric GPA was detected (data reviewed, but not shown). It should be noted that each membrane fraction used for Western blotting was isolated from the same number of normal and band 3 −/− red blood cells. However, a more precise quantitative method such as enzyme-linked immunosorbent assay (ELISA) will be required to compare the absolute amounts of membrane proteins in normal and band 3 null red blood cells.
Western blot analysis of the red blood cell membrane proteins. Red blood cells were collected from both band 3 +/+ and band 3 −/−mice and washed in PBS. Ghosts were prepared by lysis as described in Materials and Methods. Ghosts membranes were solubilized and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to nitrocellulose, Western blot analysis was performed using the ECL chemiluminescence kit as suggested by the manufacturer. Both band 3 +/+ and band 3 −/− red blood cell membranes contain protein 4.1, glycophorin C, p55, dematin, and adducin, while the complete absence of protein 4.2 and GPA is noted. The GPA panel shows the position of the dimer. No monomeric GPA was detected (data reviewed, but not shown). It should be noted that each membrane fraction used for Western blotting was isolated from the same number of normal and band 3 −/− red blood cells. However, a more precise quantitative method such as enzyme-linked immunosorbent assay (ELISA) will be required to compare the absolute amounts of membrane proteins in normal and band 3 null red blood cells.
To confirm the absence of GPA in band 3 −/− red blood cells, these cells were examined by indirect immunofluorescence after a brief exposure to Triton X-100 to allow permeabilization and penetration of the antibodies into the cells. As shown in Fig 2, no GPA signal was detected in band 3 −/− red blood cells, whereas the GPA epitopes were present in the band 3 +/+ red blood cells. Having established the absence of GPA expression on the membrane of band 3 −/− red blood cells, we next examined whether the GPA mRNA is present in the cytoplasm of early erythroblasts isolated from the spleens of band 3 −/− mice. Total RNA was reverse transcribed and GPA cDNA was amplified by PCR using GPA-specific primers (see Materials and Methods). As shown in Fig 3, a comparable amount of GPA mRNA is present in normal and band 3 −/− erythroblasts, although a more precise quantification will be required to determine the absolute amounts of GPA mRNA in normal and band 3 null red blood cells. The positive and negative controls used for the PCR amplification consisted of β globin and band 3 mRNA, respectively (Fig 3).
Immunocytochemistry of red blood cells. Red blood cells from band 3 +/+ and band 3 −/− mice were fixed onto Alcian Blue-coated coverslips with 4% paraformaldehyde. After permeabilization of the cells in a PBS solution containing 0.1% Triton-X 100 and blocking in a PBS solution with 1.0% bovine serum albumin and 1.0% goat serum, the cells were incubated with a rabbit antibody raised against the cytoplasmic domain of human GPA. The signal was detected with a goat antirabbit/rhodamine-conjugated secondary antibody. In contrast to band 3 +/+ red blood cells, band 3 −/−red blood cells do not display any detectable level of GPA on their surface.
Immunocytochemistry of red blood cells. Red blood cells from band 3 +/+ and band 3 −/− mice were fixed onto Alcian Blue-coated coverslips with 4% paraformaldehyde. After permeabilization of the cells in a PBS solution containing 0.1% Triton-X 100 and blocking in a PBS solution with 1.0% bovine serum albumin and 1.0% goat serum, the cells were incubated with a rabbit antibody raised against the cytoplasmic domain of human GPA. The signal was detected with a goat antirabbit/rhodamine-conjugated secondary antibody. In contrast to band 3 +/+ red blood cells, band 3 −/−red blood cells do not display any detectable level of GPA on their surface.
RT-PCR of mRNA isolated from spleen erythropoietic cells. Total RNA was isolated from the spleens of normal and band 3 null mice as described in Materials and Methods. cDNA was synthesized using random hexamer primers and M-MLV reverse transcriptase. Specific GPA primers were used to amplify a small fragment of the GPA cDNA. Small segments of band 3 and β globin cDNA were also amplified as negative and positive controls, respectively. The results confirm the presence of GPA mRNA indicating that the transcription of the gene takes place in band 3 −/− erythroblasts.
RT-PCR of mRNA isolated from spleen erythropoietic cells. Total RNA was isolated from the spleens of normal and band 3 null mice as described in Materials and Methods. cDNA was synthesized using random hexamer primers and M-MLV reverse transcriptase. Specific GPA primers were used to amplify a small fragment of the GPA cDNA. Small segments of band 3 and β globin cDNA were also amplified as negative and positive controls, respectively. The results confirm the presence of GPA mRNA indicating that the transcription of the gene takes place in band 3 −/− erythroblasts.
The complete absence of GPA protein in band 3 −/− red blood cells, despite an apparently normal transcription of its gene, suggested that the loss of GPA in the red blood cell membranes may be due to an abnormal synthesis and turnover of GPA polypeptides in vivo. To test this hypothesis, we measured the rate of synthesis, the stability, and the membrane incorporation of GPA in murine erythroblasts. Control and band 3 −/− erythroblasts were metabolically labeled with [35 S]methionine for 60 minutes and pulse-chased with unlabeled methionine for specified time intervals. The soluble and membrane fractions were separated and immunoprecipitated with anti-GPA antibodies. In normal erythroblasts, GPA immunoprecipitation showed that the newly synthesized protein in the soluble fraction is, as expected, approximately 5.0 kD shorter than the protein assembled in the membrane fraction (Fig 4). This size difference presumably reflects the degree of glycosylation: the newly synthesized GPA is cotranslationally inserted into the ER membrane where it gets N-glycosylated. Complete glycosylation takes place by the addition of O-linked sugars during its transit to the plasma membrane.2 49 In band 3 −/− erythroblasts, newly synthesized GPA is also detected in the soluble fraction. The amount of GPA synthesized in band 3 −/− cells during the 60-minute labeling period (ie, at time 0 minute of chase) is decreased compared with the amount in band 3 +/+ cells, but reaches normal levels after 60 minutes of chase. These results suggest that the synthesis of GPA is delayed in band 3 −/− cells. The increase in the amount of radiolabeled GPA after 60 minutes of chase likely represents the completion and release of nascent polypeptides synthesized during the pulse labeling period. Once the synthesis is complete, the newly synthesized GPA assembles stably in the membrane of band 3 +/+ cells. In contrast, the newly synthesized GPA in band 3 −/− cells is not incorporated into the membrane and is presumably degraded in the cytoplasm (Fig 4). At this stage, the basis for the delayed synthesis of GPA in band 3 −/− erythroblasts is not known. A reasonable speculation is that the presence of band 3 has a modulatory effect on the synthesis and turnover of GPA in vivo.
Synthesis, stability, and membrane incorporation of GPA in mouse erythroblasts. Band 3 +/+ and band 3 −/− erythroblasts were labeled with [35S]methionine for 60 minutes and then chased with unlabeled methionine for 0, 60, and 120 minutes. Thereafter, the cells were lysed and separated into soluble (ER) and insoluble (membrane) fractions. The identity of the membrane fraction derived from band 3 −/− erythroblasts was confirmed by immunoprecipitation of protein 4.1 (data reviewed, but not shown). Equal volumes of each sample were immunoprecipitated using anti-GPA antibodies and the immunoprecipitates were analyzed by SDS-PAGE. The immunoprecipitation conditions were similar to those described previously.46 The gels were processed for fluorography. The immunoprecipitated bands in the autoradiogram correspond to the position of GPA dimer. The absence of GPA in the membrane fraction of band 3 −/− erythroblasts shows that despite the synthesis of GPA in band 3−/− erythroblasts, GPA is not recruited to the plasma membrane and is presumably degraded rapidly in the cytoplasm. The results of immunoprecipitated GPA were confirmed by three independent experiments. GPa, partially glycosylated glycophorin A; GPA, completely glycosylated glycophorin A.
Synthesis, stability, and membrane incorporation of GPA in mouse erythroblasts. Band 3 +/+ and band 3 −/− erythroblasts were labeled with [35S]methionine for 60 minutes and then chased with unlabeled methionine for 0, 60, and 120 minutes. Thereafter, the cells were lysed and separated into soluble (ER) and insoluble (membrane) fractions. The identity of the membrane fraction derived from band 3 −/− erythroblasts was confirmed by immunoprecipitation of protein 4.1 (data reviewed, but not shown). Equal volumes of each sample were immunoprecipitated using anti-GPA antibodies and the immunoprecipitates were analyzed by SDS-PAGE. The immunoprecipitation conditions were similar to those described previously.46 The gels were processed for fluorography. The immunoprecipitated bands in the autoradiogram correspond to the position of GPA dimer. The absence of GPA in the membrane fraction of band 3 −/− erythroblasts shows that despite the synthesis of GPA in band 3−/− erythroblasts, GPA is not recruited to the plasma membrane and is presumably degraded rapidly in the cytoplasm. The results of immunoprecipitated GPA were confirmed by three independent experiments. GPa, partially glycosylated glycophorin A; GPA, completely glycosylated glycophorin A.
Band 3 serves as a major attachment site between the spectrin-actin–based skeleton and the plasma membrane. This physical membrane-skeleton coupling is largely achieved by the formation of a complex between band 3, ankyrin, β spectrin, and protein 4.2 (for review, see Tanner12 and Low13). In addition, it is believed that band 3 also plays a role in the attachment of the spectrin-actin junctional complexes to the membrane via its binding to protein 4.1.50,51 Because spectrin-actin junctional complexes also contain proteins such as adducin, dematin, and p55, we examined the presence of these proteins in band 3 −/− red blood cell membranes. As shown in Fig 1, Western blotting demonstrated the presence of protein 4.1, glycophorin C, p55, dematin, and adducin in band 3 −/− red blood cell membranes. These results indicate that the presence of band 3 is not required for the assembly of the junctional complex proteins on the red blood cell membrane and supports the notion that the ternary complex consisting of glycophorin C, protein 4.1, and p55 provides an independent site for the attachment of spectrin-actin junctional complexes in vivo.43 52 In contrast, the concomitant loss of protein 4.2 in band 3 −/− red blood cells suggests that another dominant site for the attachment of the spectrin-ankyrin complex to the plasma membrane may be provided by the ternary complex consisting of band 3, GPA, and protein 4.2.
Because of the unavailability of mature red blood cells completely deficient in the band 3 protein, previous studies have focused on studying the effects of GPA loss on the expression, biosynthesis, and processing of band 3. The complete loss of GPA in naturally occurring En(a-) and MkMk red blood cells is not associated with any significant phenotypic abnormality.8-10 Nevertheless, the loss of GPA was shown to modulate the extent of the N-glycan chain on band 3 in GPA-deficient red blood cells.8,25,53 These observations led to the suggestion that the absence of GPA may delay the export of band 3 from the golgi apparatus, thus extending the length of band 3 glycosylation during erythropoiesis. Similarly, the sulfate transport properties of band 3 were shown to be impaired in red blood cells lacking GPA, suggesting a reduced affinity of band 3 for sulfate anions in the absence of GPA.53 Finally, in the Xenopus oocyte expression system, it was shown that GPA facilitates the expression of band 3 on the membrane and enhances the anion transport function of band 3 in a heterologous expression system.54 Together, these previous studies clearly suggest an important role for GPA in the regulation of band 3 functions in vivo and provide convincing evidence for the physical interaction of these proteins in the red blood cell membrane. However, it is relevant to mention here that human erythroleukemic K562 cells do not synthesize band 3 (AE1) protein, yet they express a surface membrane glycoprotein that is identical or closely similar to GPA.55,56 This observation suggests that other alternate mechanisms may exist in K562 cells that compensate for the absence of band 3. Indeed, K562 cells are reported to express a band 3-like glycoprotein termed GP105 that may associate with GPA and functionally substitute for the AE1 protein.55 56
In conclusion, the recent development of band 3 −/− mice by us and others40,41 allowed us to study the effect of band 3 loss on GPA and other red blood cell membrane proteins. Our findings showing complete loss of GPA in band 3 −/− mice provide, for the first time, an unequivocal proof that the biosysthesis and stability of GPA is dependent on the presence of band 3 in vivo. Our results also suggest that band 3, GPA, and protein 4.2 may form a ternary complex in the red blood cell membrane. Based on these findings and other published evidence,32-39 we propose that band 3 not only interacts with GPA in the membrane of mature red blood cells, but also plays an essential role in the processing and transport of intracellular GPA during erythropoiesis. This putative “chaperone-like” function of band 3 is consistent with similar paradigms established for other membrane proteins.57 58 The loss of GPA and protein 4.2 in band 3 −/− red blood cells raises interesting questions about the role of band 3 and protein 4.2 on the kinetics of transport, assembly, expression, and processing of GPA in the red blood cell membrane.
ACKNOWLEDGMENT
The authors thank Iva Smockova for her technical help in the biosynthetic experiments, Jennifer Wu for her expertise in alignment analysis and editorial assistance, Donna-Marie Mironchuk for the art work, and Dr Shih-Chun Liu for helpful discussions.
H.H. and T.H. contributed equally to this report.
Supported by Grants No. HL 51445 and CA66263 (to A.H.C.) and KO8 HL02720 (to H.H.) from the National Institutes of Health, Bethesda, MD. A.H.C. is an established investigator of the American Heart Association.
Address reprint requests to Athar H. Chishti, PhD, ACH4 Building, St. Elizabeth's Medical Center, 736 Cambridge St, Boston, MA 02135.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Fig. 4. Synthesis, stability, and membrane incorporation of GPA in mouse erythroblasts. Band 3 +/+ and band 3 −/− erythroblasts were labeled with [35S]methionine for 60 minutes and then chased with unlabeled methionine for 0, 60, and 120 minutes. Thereafter, the cells were lysed and separated into soluble (ER) and insoluble (membrane) fractions. The identity of the membrane fraction derived from band 3 −/− erythroblasts was confirmed by immunoprecipitation of protein 4.1 (data reviewed, but not shown). Equal volumes of each sample were immunoprecipitated using anti-GPA antibodies and the immunoprecipitates were analyzed by SDS-PAGE. The immunoprecipitation conditions were similar to those described previously.46 The gels were processed for fluorography. The immunoprecipitated bands in the autoradiogram correspond to the position of GPA dimer. The absence of GPA in the membrane fraction of band 3 −/− erythroblasts shows that despite the synthesis of GPA in band 3−/− erythroblasts, GPA is not recruited to the plasma membrane and is presumably degraded rapidly in the cytoplasm. The results of immunoprecipitated GPA were confirmed by three independent experiments. GPa, partially glycosylated glycophorin A; GPA, completely glycosylated glycophorin A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/6/10.1182_blood.v91.6.2146/2/m_blod4060604.jpeg?Expires=1763749432&Signature=C3fHp5B~oZJX7r3M8~bV47bCliW1tbXwpEC1KD27VKfwKzhpUrJOsBhEzYTGP8Gy-YfJIwP2hqjnAM~ktSiggilVHynHVmAn2aOJlbs4KRW7Bec0HStk7f5cPKxdu2eeqxPNQbdLvhdluzF7A0iBnmUJRvsy8qTcVW6A27UucgWQIY-wWiA1HAE5EAK0LvLN5uOo3Sh5p5DJm3WiKDWy7fc6lX1aFj4mqukA0Ej04dqh7Rgt~7g1vj73dPBZ9-RP4agB42L--jtbjnMfe5h86zWNc9nY9zZjc-C7mEBsRwhBEtpHeHC45Xf8pXemmnC4XFmKF5fwn7Y~iyFddc7PtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 4. Synthesis, stability, and membrane incorporation of GPA in mouse erythroblasts. Band 3 +/+ and band 3 −/− erythroblasts were labeled with [35S]methionine for 60 minutes and then chased with unlabeled methionine for 0, 60, and 120 minutes. Thereafter, the cells were lysed and separated into soluble (ER) and insoluble (membrane) fractions. The identity of the membrane fraction derived from band 3 −/− erythroblasts was confirmed by immunoprecipitation of protein 4.1 (data reviewed, but not shown). Equal volumes of each sample were immunoprecipitated using anti-GPA antibodies and the immunoprecipitates were analyzed by SDS-PAGE. The immunoprecipitation conditions were similar to those described previously.46 The gels were processed for fluorography. The immunoprecipitated bands in the autoradiogram correspond to the position of GPA dimer. The absence of GPA in the membrane fraction of band 3 −/− erythroblasts shows that despite the synthesis of GPA in band 3−/− erythroblasts, GPA is not recruited to the plasma membrane and is presumably degraded rapidly in the cytoplasm. The results of immunoprecipitated GPA were confirmed by three independent experiments. GPa, partially glycosylated glycophorin A; GPA, completely glycosylated glycophorin A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/6/10.1182_blood.v91.6.2146/2/m_blod4060604.jpeg?Expires=1763855233&Signature=BtNRKUXyFG5oHRawNkUeKBmCKDGmoOOIQPXds0Geue8Yxgmg-KQVEmlO1LyYzYsiNLUvV3YFi~XKL2zCpnwkSUoc5Ie0NJL3rzadLDxG2AMgxAAsmFdAHpAXK7eKBnPVJIECBFQF-TSOco3pKNewFYoaXS3U8fWCvEe-9qk4OAGMs3~S3Tqj7URyapKgn4hmDVdJCeQ1N0ZibLey9cPM7DGnx3uYKJtFsG-BOxyy0Uiuz~WH4Sb~nJI4fSpsT9AuaMw~TkhvIjxmqtRP~W1l4Hwll0ElUkVV2VdIc~ceYu0nntX~fWbH0Fdbc4pL8JQfEMCNPnP5j5dqU5pABqK9LA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)