Abstract
There are no readily applicable methods to routinely assess thrombosis risk and treatment response in thrombocytosis. Reticulated platelets (RP) define the most recently released platelets in the circulation, and the RP% has been shown to estimate platelet turnover in thrombocytopenic states. We examined whether increased RP values were associated with thrombotic complications in thrombocytosis. Platelet count, RP%, and absolute RP count were measured at presentation in 83 patients with chronic or transient thrombocytosis, 46 patients with deep vein (DVT) or arterial (ART) thrombosis and normal platelet counts, and 83 healthy controls with normal platelet counts. Chronic thrombocytosis patients presenting with thrombosis (n = 14) had significantly higher RP% (14.7% ± 10.1%, mean ± SD) than asymptomatic chronic thrombocytosis patients (n = 23, RP% = 3.4% ± 1.8%), healthy controls (3.4% ± 1.3%), DVT patients (n = 21, 3.8% ± 2.1%), or ART patients (n = 25, 4.5% ± 4.1%, P < .05 for all comparisons). Chronic thrombocytosis patients with thrombosis also had significantly higher absolute RP counts than asymptomatic chronic thrombocytosis patients (98 ± 64 × 109/L [range, 54 to 249 × 109/L] v 30 ± 13 × 109/L [range, 11 to 51 × 109/L]; P = .0004), whereas healthy controls, DVT, and ART patients had similarly low absolute RP counts (6 ± 6 × 109/L, 9 ± 7 × 109/L, and 11 ± 7 × 109/L, respectively; P > .49). The RP% and absolute RP counts remained significantly higher in chronic thrombocytosis patients with thrombosis when patients were further subdivided into primary myeloproliferative disorders versus secondary thrombocytosis. Similarly elevated RP percentages and absolute counts were also noted in transient thrombocytosis patients with thrombosis (n = 6, 11.5% ± 4.4% and 90 ± 46 × 109/L, respectively) when compared with asymptomatic transient thrombocytosis patients (n = 40, 4.5% ± 2.7% and 35 ± 16 × 109/L, respectively) and to all control groups (P < .05 for all comparisons). In addition, 7 of 8 thrombocytosis patients who were studied before developing symptoms of thrombosis had elevated absolute RP counts compared with only 1 of 63 thrombocytosis patients who remained asymptomatic. Follow-up studies in seven chronic thrombocytosis patients showed that successful aspirin treatment of symptomatic recurrent thrombosis significantly reduced the RP% from 17.1% ± 10.9% before therapy to 4.8% ± 2.0% after therapy; absolute RP counts decreased from 102 ± 67 × 109/L to 26 ± 10 × 109/L (P < .01 for both). We conclude that thrombosis in the setting of an elevated platelet count is associated with increased platelet turnover, which is reversed by aspirin therapy. Measurement of reticulated platelets to assess platelet turnover may be useful in evaluating both treatment response and thrombotic risk in thrombocytosis.
PATIENTS WITH PRIMARY thrombocytosis associated with myeloproliferative disorders (MPD) may have hemorrhagic1,2 and thrombotic complications that are presumed to relate to either abnormal platelet function,3-6the increased number of circulating platelets, or both.7-9Secondary or reactive thrombocytosis has also been linked to thrombotic complications in various settings, although some investigators believe that the evidence for a direct association is minimal in this patient group.7,10-14 However, in either group, specific risk factors for thrombotic or bleeding complications in the setting of an increased platelet count are relatively unclear. In at least one study, higher platelet counts appeared to correlate best with bleeding, whereas lower (but still elevated) counts tended to be associated with thrombosis15; nonetheless, threshold counts for intervention are not well defined. Platelets from primary thrombocytosis patients show abnormal arachadonic acid metabolism and agonist response,16-18 but this finding may not predict either thrombosis or bleeding.16,18 In vivo platelet activation has been demonstrated during thrombosis in both primary and secondary thrombocytosis,6,19-21 and both platelet activation and decreased isotopic platelet half-life have been noted in MPD patients with erythromelalgia.22 23 However, these specialized laboratory measurements are not readily accessible; hence, examination of a simpler measurement of platelet turnover might provide both mechanistic and clinically valuable information on the occurrence of thrombosis in the setting of thrombocytosis, regardless of the underlying cause.
Similar to red blood cell reticulocytes,24 reticulated platelets (RP) are defined by their increased RNA content.25,26 In animals, RP appear to be the youngest circulating platelets,27 and there is evidence in a dog model that the youngest platelets are also the most functionally active.28 Thrombocytopenia due to decreased platelet survival has been shown to result in an increased RP%.26,29-31 Furthermore, RP may also reflect increased platelet turnover in the setting of a normal platelet count, because women who went on to develop pre-eclampsia had an elevated RP% before they became symptomatic or developed thrombocytopenia.32Therefore, RP measurements might provide a rapid and simple measure of platelet turnover in patients with thrombocytosis. RP values might, in turn, reflect increased platelet consumption during the evolution of thrombosis and/or as a prelude to the development of thrombosis. We examined whether increased reticulated platelet values were associated with thrombotic complications in patients with thrombocytosis and compared this group with healthy controls and patients presenting with deep vein or arterial thrombosis in the setting of a normal platelet count.
MATERIALS AND METHODS
Patients.
This study was approved by the Yale University Human Investigation Committee. All patients were consecutively enrolled. Thrombocytosis patients were included if they had a platelet count greater than 600 × 109/L.1 2 Patients with deep vein thrombosis (DVT) or arterial thrombosis (ART) were included if their platelet count was between 150 and 450 × 109/L. Patients were excluded if they had prolonged coagulation studies or if they were receiving anticoagulant or cytotoxic therapy, growth factors, or medications affecting platelet function. A healthy volunteer on no medications with a platelet count between 150 and 450 × 109/L was included as a simultaneous control in each study. Ninety-eight thrombocytosis patients, 48 ART patients, and 25 DVT patients were initially enrolled. Forty-two subjects were subsequently excluded because they were receiving coumadin (n = 5), chemotherapeutic agents (n = 8), recombinant growth factors (n = 6), or aspirin (n = 23) at enrollment, leaving 83 thrombocytosis patients, 25 ART patients, and 21 DVT patients evaluable for the study.
Patient records were reviewed by an investigator blinded to all laboratory results. Thrombocytosis was defined as transient if the platelet count was within the normal range (<450 × 109/L) within 4 months before or after the study sample and the patient was not receiving cytotoxic therapy. Chronic thrombocytosis was defined as a platelet count persistently greater than 600 × 109/L over the same time period and was additionally confirmed by an independent clinical hematology consultant reviewing the patient's history. Patients with chronic thrombocytosis were subdivided into (1) primary MPD-associated thrombocytosis due to polycythemia vera (PV) or essential thrombocythemia (ET)1,9or (2) secondary thrombocytosis associated with diseases other than primary hematopoietic disorders.33 All thrombotic events were confirmed by (1) direct observation at surgery; (2) angiography, ultrasonography, or magnetic resonance imaging; (3) classic symptoms of erythromelalgia; or (4) electrocardiogram and creatinine kinase documentation of a myocardial infarction.
Blood sampling and preparation.
Blood samples were drawn at presentation to the hospital or clinic, before any medical or surgical intervention was begun. Fourteen patients with chronic thrombocytosis also had follow-up blood studies performed at the first new visit after their initial study. After informed consent, venous blood was drawn into EDTA (1.5 mg/mL); a complete blood count (CBC) was confirmed by manual counts. Platelets were prepared from blood as previously described.34Briefly, blood was centrifuged to obtain platelet-rich plasma; platelets were washed with Tyrodes buffer and fixed in 1% paraformaldehyde at 4°C for 1 hour. After fixation, platelets were washed and resuspended at 200 × 109/L.
Labeling.
One hundred microliters of the platelet suspension was labeled with phycoerythrin (PE)-anti-gpIIb/IIIa (P2; Coulter, Hialeah, FL) and then washed and incubated with thiazole orange (Retic-COUNT; Becton Dickinson Immunocytometry Systems, San Jose, CA) at 22°C for 60 minutes (final concentration, 90% vol/vol).31,33 35Platelet samples pretreated with ribonuclease showed loss of thiazole fluorescence, as previously described.
Flow cytometry.
Measurement of platelet thiazole fluorescence was performed on a FACScan (Becton Dickinson) flow cytometer with compensation settings to prevent PE fluorescence bleedover into thiazole orange fluorescence; quantitation of the RP% was performed as previously described.31,34 35 The coefficient of variation for repeated RP measurements of the same sample was less than 5%. The absolute RP count was calculated by multiplying the RP% by the platelet count.
Statistics.
Statistical analysis was performed with Statgraphics Plus (Manugistics, Rockville, MD). Comparisons between groups were examined for significance (P < .05) with the Student's t-test or, if distribution fitting by χ2 analysis showed a non-normal distribution, the Mann-Whitney U-test for nonparametric values. The strength of associated risk factors for a thrombotic complication was estimated by calculation of the odds ratio and 95% confidence intervals (95% CI).
RESULTS
Patient demographics.
The 83 thrombocytosis patients studied included 48 women and 35 men ranging from 18 to 84 years of age, with a median age of 52 years. There were 37 subjects (24 women and 13 men) with chronic thrombocytosis; of these, 12 had chronic primary thrombocytosis associated with PV (n = 3) or ET (n = 9; Table 1). None of the primary thrombocytosis patients had had a prior thrombotic event, and all were previously untreated (except for phlebotomy in the 3 patients with PV) at enrollment. Twenty-five patients had chronic secondary thrombocytosis associated with sickle cell disease (n = 7), remote (>1 year) splenectomy (n = 8), or chronic inflammatory diseases (n = 10; Table 1). There were 46 subjects (23 women and 23 men) with transient thrombocytosis (Table 1) associated with acute infectious or inflammatory disease (n = 24), massive hemorrhage (n = 8), malignancy (n = 9), or recent (<12 weeks) splenectomy for trauma (n = 5), all conditions previously reported to be associated with thrombocytosis.10 36-41
The 14 chronic thrombocytosis patients with follow-up studies had blood samples drawn between 4 and 30 weeks after enrollment. One patient with ET was receiving aspirin and hydroxyurea and was excluded from follow-up analysis. Of the remaining 13 patients, 5 of 7 patients with chronic primary thrombocytosis (4 with ET and 1 with PV) and 2 of 6 patients with chronic secondary thrombocytosis (both postsplenectomy) were receiving aspirin alone; the remaining 6 patients (1 with PV, 1 with ET, 2 with sickle cell disease, and 2 postsplenectomy) were not receiving any medication at follow-up.
Twenty of the 83 thrombocytosis patients (24%) had thrombotic events; 8 of these patients had blood studies drawn more than 24 hours before they became symptomatic, whereas 12 had blood studies drawn simultaneous with or shortly (<24 hours) after the onset of thrombotic symptoms. Age and gender distribution did not differ significantly between patients with and without thrombosis in any of the chronic or transient thrombocytosis groups (P > .27 for all comparisons). The average age for all thrombocytosis patients with (n = 20) and without (n = 63) thrombosis was 50 ± 21 (SD) years and 52 ± 17 years, respectively (P = .90).
Of the 21 DVT patients, there were 12 women and 9 men with a median age of 53 years (range, 22 to 76 years), which was not different from all thrombocytosis patients (P = .74). All 21 DVT patients presented within 36 hours after developing symptoms of thrombosis, and all had blood studies drawn at presentation before therapy was begun. Of the 25 ART patients, there were 12 women and 13 men with a median age of 62 years (range, 27 to 81 years), which did not differ from the thrombocytosis group (P = .61). Ten ART patients had myocardial infarction, whereas 15 had thrombosis involving the middle cerebral, iliac, femoral, or popliteal arteries; all ART patients had blood studies drawn less than 12 hours after developing symptoms and before therapy was begun.
Chronic thrombocytosis (n = 37).
Seven of the 12 individuals (58%) with chronic primary (MPD-associated) thrombocytosis had arterial/arteriolar thrombotic complications, including 5 patients (4 ET and 1 PV) with erythromelalgia and 2 patients (ET) with brachial and superior mesenteric artery thromboses, respectively. Seven of 25 patients (28%) with chronic secondary thrombocytosis had acute thrombotic events; these included DVT in 1 of 7 sickle cell (SS) disease patients; thromboses of the brachial and middle cerebral arteries, respectively, in 2 of 8 patients with remote splenectomy; and thromboses of the femoral, popliteal, and splenic arteries, and infrarenal aorta, respectively, in 4 of 10 patients with chronic inflammatory diseases. These inflammatory disease patients carried diagnoses of iron deficiency anemia (n = 1), rheumatoid arthritis (n = 1), and diabetes mellitus (n = 2).
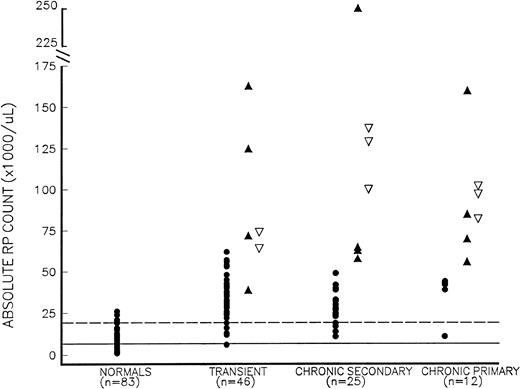
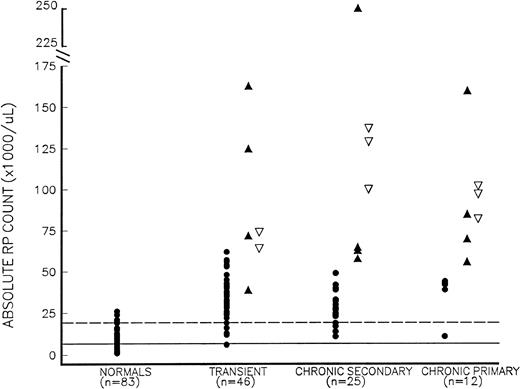
The 14 chronic thrombocytosis patients with thrombotic events had a significantly higher RP% (14.7% ± 10.1%, mean ± SD) than both asymptomatic chronic thrombocytosis subjects (n = 23, 3.4% ± 1.8%) and normal controls (3.4% ± 1.3%, P< .002 for both comparisons); the RP% in asymptomatic chronic thrombocytosis patients did not differ significantly from control values (P = .82). Patients with thrombosis also had a significantly higher absolute RP count, without any overlap, than those without thrombosis, 98 ± 64 × 109/L (range, 54 to 249 × 109/L) versus 30 ± 13 × 109/L (range, 11 to 51 × 109/L; P= .0004). When chronic thrombocytosis patients were further subdivided into primary and secondary causes of thrombocytosis (Table 2 and Fig 1), both the RP% and the absolute RP counts remained significantly higher in patients with thrombosis than in asymptomatic subjects. Furthermore, when symptomatic chronic thrombocytosis patients (primary and secondary) were subdivided into those with erythromelalgia and those with larger arterial or venous events, both the RP% and absolute RP counts remained higher in these subsets compared with asymptomatic chronic thrombocytosis patients (P < .05 for all comparisons).
Absolute RP counts (RP% × platelet count) in thrombocytosis. Thrombocytosis patients include those who presented with symptomatic thrombosis (▴), those studied more than 24 hours before developing thrombosis (▿), and those who remained asymptomatic (•), as well as normal controls (•) with normal platelet counts. Thrombocytosis patients are further subdivided into those with (1) transient thrombocytosis; (2) chronic secondary thrombocytosis; and (3) chronic primary (MPD-associated) thrombocytosis. The mean and mean + 2 SD values for normal controls are shown as solid and dotted lines, respectively.
Absolute RP counts (RP% × platelet count) in thrombocytosis. Thrombocytosis patients include those who presented with symptomatic thrombosis (▴), those studied more than 24 hours before developing thrombosis (▿), and those who remained asymptomatic (•), as well as normal controls (•) with normal platelet counts. Thrombocytosis patients are further subdivided into those with (1) transient thrombocytosis; (2) chronic secondary thrombocytosis; and (3) chronic primary (MPD-associated) thrombocytosis. The mean and mean + 2 SD values for normal controls are shown as solid and dotted lines, respectively.
There was a trend towards significantly higher platelet counts in all chronic thrombocytosis subjects with thrombosis (871 ± 218 × 109/L) compared with patients who did not have thrombosis (729 ± 131 × 109/L; P = .08). This trend was due to the platelet counts being significantly higher in chronic secondary thrombocytosis patients with thrombosis (range, 625 to 1,250 × 109/L) than in asymptomatic patients (range, 600 to 843 × 109/L; P = .045; Table 2); platelet counts did not differ significantly between chronic primary thrombocytosis patients with and without thrombosis (range, 653 to 851 × 109/L v 688 to 1,120 × 109/L, respectively; P = .91; Table 2).
Transient thrombocytosis (n = 46).
Six patients with transient thrombocytosis had acute thrombotic events. One patient with underlying inflammatory disease had a large arterial thrombosis, and two patients with inflammatory disease and malignancy, respectively, had myocardial infarctions. DVT occurred in 2 patients with malignancy and in 1 patient postsplenectomy. Similar to chronic thrombocytosis, patients with transient thrombocytosis and thrombosis had significantly higher RP% and absolute RP counts than asymptomatic patients (Table 2) or normal controls (P < .01 for both). By contrast, platelet counts did not differ (P = .17) between thrombotic and asymptomatic patients with transient thrombocytosis (Table 2), and the RP% in asymptomatic transient thrombocytosis subjects was also not significantly different (P = .48) from normal controls (Table 2).
Reticulated platelet studies drawn before thrombosis.
Seventy-one of the 83 thrombocytosis patients had blood studies drawn when they were asymptomatic (Fig 1), and 8 of 71 (11%) subsequently developed thrombotic complications (all within 16 days of blood sampling). Six of these 8 (75%) symptomatic patients had an RP% greater than 6.0% (the mean + 2 SD value for normal controls), whereas only 10 of 63 patients (16%) who remained asymptomatic had an RP% greater than 6%; therefore, the odds ratio for developing a thrombosis with an RP% greater than 6% was 10.3 (3.83 to 46.2, 95%CI). By contrast, only 1 of 8 (13%) thrombosis patients had a platelet count greater than 1,000 × 109/L compared with 13 of 63 (21%) asymptomatic patients, yielding an odds ratio of 0.51 (0.07 to 3.81, 95% CI). For an absolute RP count greater than 60 × 109/L (the product of an RP% >6% and a platelet count >1,000 × 109/L), 7 of 8 (88%) thrombocytosis patients who subsequently developed thrombosis exceeded this value compared with only 1 of 63 (2%) asymptomatic patients (Fig 1). The odds ratio for thrombosis with an absolute RP count greater than 60 × 109/L was 56.0 (7.89 to 398, 95% CI).
DVT and ART patients with normal platelet counts.
The 21 patients with DVT and the 25 patients with ART had platelet counts (272 ± 87 × 109/L and 286 ± 95 × 109/L), RP% (3.8% ± 2.1% and 4.5% ± 4.1%), and absolute RP counts (9 ± 7 × 109/L and 11 ± 7 × 109/L), respectively, that did not differ from healthy controls (P > .49 for all comparisons; Table 3). Furthermore, the RP% of both DVT and ART patients was similar to asymptomatic thrombocytosis patients (P > .30 for all comparisons); by contrast, the RP% in both DVT and ART patients was significantly lower than the RP% in all symptomatic thrombocytosis subgroups (Table 2) as well as in the subgroup of all thrombocytosis patients with localized venous or arterial thrombosis (n = 15, 14.9% ± 10.1%; P < .05 for all comparisons).
Follow-up studies in chronic thrombocytosis (n = 13).
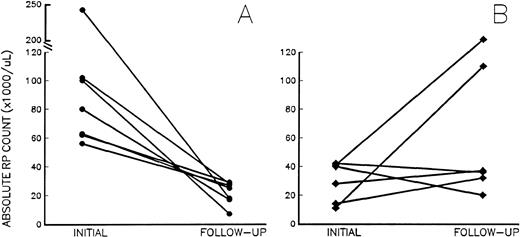
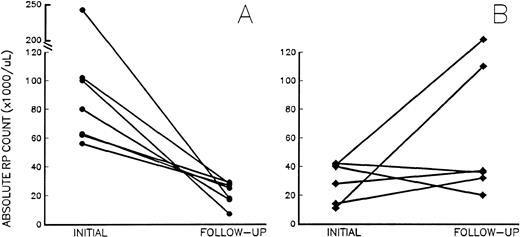
At follow-up, the 7 patients receiving aspirin had a significant decrease in their RP% from 17.1% ± 10.9% to 4.8% ± 2.0% (P = .006), and the absolute RP counts decreased from 102 ± 67 × 109/L to 26 ± 10 × 109/L (Fig 2A; P = .007), despite platelet counts remaining unchanged (687 ± 211 × 109/L to 635 ± 191 × 109/L;P = .42). Of note, after the inititation of aspirin, the absolute RP counts in treated patients ranged from 7 to 35 × 109/L, below the arbitrary risk level of 60 × 109/L; erythromelalgia resolved in 3 MPD patients treated with aspirin, and none of the aspirin-treated patients had thrombosis during the follow-up period. By contrast, platelet counts, RP%, and absolute RP counts did not change in 4 of the 6 untreated patients (Fig2B) who were asymptomatic at follow-up. However, 2 untreated patients with sickle cell disease and PV suffered from subsequent pulmonary embolism and superior mesenteric artery thrombosis, respectively. Although follow-up studies were not obtained before becoming symptomatic, absolute RP counts on admission had increased dramatically in both patients (Fig 2B).
Absolute RP counts (RP% × platelet count) in chronic thrombocytosis patients (A) before and after treatment with aspirin (•) and (B) at baseline and follow-up in untreated chronic thrombocytosis patients (⧫). Initial (baseline) values were drawn at enrollment into the study; follow-up studies were drawn at the first new visit after enrollment. The interval between studies ranged from 4 to 23 weeks and 7 to 30 weeks in treated and untreated subjects, respectively.
Absolute RP counts (RP% × platelet count) in chronic thrombocytosis patients (A) before and after treatment with aspirin (•) and (B) at baseline and follow-up in untreated chronic thrombocytosis patients (⧫). Initial (baseline) values were drawn at enrollment into the study; follow-up studies were drawn at the first new visit after enrollment. The interval between studies ranged from 4 to 23 weeks and 7 to 30 weeks in treated and untreated subjects, respectively.
DISCUSSION
This study has used a rapid and accessible method of evaluating platelet kinetics to show that increased percentages and absolute numbers of RP are highly associated with thrombosis in patients with thrombocytosis. All subsets of thrombocytosis patients with thrombosis had significantly higher RP% compared with patients without thrombosis, and all chronic thrombocytosis patients with thrombosis had a greater absolute number of RP than the highest value observed in asymptomatic subjects. Treatment with aspirin uniformly caused a decrease in the RP% and absolute RP counts concomitant with complete symptomatic improvement in patients with erythromelalgia and the absence of recurrent thrombosis in all treated patients. These data suggest that changes in platelet turnover in the setting of an elevated platelet count may be reflected by RP values, and such changes could be correlated with successful antithrombotic therapy. Moreover, when asymptomatic thrombocytosis patients were studied, an elevated RP% and absolute RP count were more often associated with subsequent development of a thrombotic complication, with respective odds ratios of 10.3 and 56.0. The positive predictive values for developing symptomatic thrombosis with an RP% greater than 6% or an absolute RP count greater than 60 × 109/L were 38% and 88%, respectively. Therefore, although our patient numbers are small and require confirmation in a prospective manner, these data further suggest that changes in RP values might reflect increased thrombotic risk in thrombocytosis. A larger prospective study is required to determine whether observing serial RP measurements over time in asymptomatic thrombocytosis patients would reliably produce an adequate interval between an increase in RP values and the development of symptomatic thrombotic events that would allow for earlier, and possibly more successful, intervention.42-44
Reticulated platelets have been shown to be the youngest circulating platelets in animals,27,45 and increased RP percentages appear to reflect increased platelet turnover in humans with destructive thrombocytopenia.26,29,30,35 Although RP have not been formally studied with respect to platelet survival in thrombocytosis, this study's findings of normal RP% in asymptomatic thrombocytosis patients is consistent with previous reports of normal platelet survival in asymptomatic patients with reactive thrombocytosis or ET.22 Furthermore, thrombosis in thrombocytosis patients has been associated with increased platelet turnover; MPD patients with erythromelalgia had decreased isotopic platelet survival that improved after aspirin therapy, suggesting that enhanced platelet activation has a role in this phenomenon.22,23 In fact, increased levels of circulating activated platelets or platelet aggregates have been noted in MPD patients with thrombosis.6,17,20 23 Our current study would support the hypothesis that an aspirin-sensitive platelet pathology is partly responsible for the simultaneous occurrence of thrombosis and increased platelet turnover, because we demonstrated a decrease in the RP% and absolute RP count in those patients who received aspirin therapy. Thus, RP measures may provide a noninvasive, readily applicable means of monitoring the response to therapy in thrombocytosis.
The increased RP values in thrombocytosis patients with thrombus may reflect increased platelet turnover due to loss of platelets into large arterial or smaller, but more widespread, arteriolar clots (erythromelalgia).46 Alternatively, increased RP measurements may reflect an abnormal platelet physiology3,38(perhaps aspirin-sensitive) causing increased platelet adhesion to endothelium, leukocytes,47,48 or other platelets and subsequent removal of those platelets from the circulation without necessarily forming a true clot. Indeed, the latter explanation has been previously cited in studies showing increased platelet turnover in asymptomatic ET patients.22 Although the fact that some thrombocytosis patients in the current study showed increased RP values before becoming symptomatic similarly suggests the latter mechanism, it is also possible that the increase in platelet turnover in these patients was the result of subclinical thrombosis. However, against this possibility is the fact that we did not find increased RP percentages in symptomatic patients presenting with DVT or ART and normal platelet counts as compared with normal controls. Nonetheless, an alternate explanation for this finding may be that the arteriolar/arterial platelet deposition in thrombocytosis is widespread enough to produce increased platelet turnover, as compared with the relatively localized venous or arterial thrombosis associated with normal platelet counts. Even with these caveats, we favor the explanation that thrombocytosis patients who are predisposed to thrombosis13 have an underlying platelet pathology associated with increased platelet turnover that is correctable by aspirin therapy. This latter hypothesis has been suggested by several other studies49 50 and is further supported by our finding that localized venous or arterial thrombosis in thrombocytosis patients was associated with a higher RP% than in DVT or ART patients with normal platelet counts.
We found a 58% incidence of arterial/arteriolar thrombotic events in untreated MPD patients, whereas patients with chronic secondary thrombocytosis had a 28% incidence of thrombotic phenomena that occurred primarily in the large arterial circulation. Most studies would suggest that MPD platelets have an abnormal physiology,3 whereas in reactive thrombocytosis, thrombotic risk may be based more on the specific setting associated with the elevated platelet count.10 The incidence of thrombotic complications in all patients with thrombocytosis (including both primary and secondary causes) has been reported to range from 11% to 80%,2,5,51 and some series report as high as a 20% incidence of fatal thrombotic events,16 52 suggesting that there are patient subsets with a high risk of thrombotic complications. The current study was not designed a priori as an epidemiologic survey and hence cannot formally address the question of whether secondary thrombocytosis is significantly prothrombotic. Patients in this study were identified sequentially on the basis of CBC with platelet counts ≥600 × 109/L received by our hematology laboratory; it is relevant to note that less than 25% of the CBC studies in our laboratory are from outpatients, and more than 80% of these outpatient samples are from specialty referral clinics. Thus, our laboratory sampling is biased toward a more ill and complicated patient population, and this bias may be responsible for the high incidence of thrombotic complications in chronic secondary thrombocytosis patients. However, regardless of the pathophysiology responsible for symptomatic thrombosis, this study found that increased platelet turnover in all subsets of thrombocytosis patients was associated with thrombosis.
In summary, thrombocytosis is associated with normal percentages of reticulated platelets when patients are free of thrombotic complications; in the perithrombotic period and specifically in a subset of asymptomatic patients who subsequently developed thrombosis, the percentage and absolute number of reticulated platelets were elevated, consistent with increased platelet turnover. By contrast, increased RP values were not seen in venous or arterial thrombosis patients with normal platelet counts. An aspirin-sensitive pathophysiology may be responsible for the association of increased platelet turnover with symptomatic thrombocytosis, because aspirin therapy resulted in lower RP values and correlated with symptomatic improvement. Reticulated platelet measurements may prove useful for monitoring therapeutic responses in patients with thrombocytosis and perhaps in stratifying those patients at risk for thrombotic complications.
Supported by National Institutes of Health Grants No. HL02668 (H.M.R.) and HL47193 (B.R.S.) and by an American Heart Association Clinician-Scientist Award (C.S.R.).
Address reprint requests to Henry M. Rinder, MD, Department of Laboratory Medicine, Yale University School of Medicine, PO Box 208035, New Haven, CT 06520-8035.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.