Abstract
Bone marrow is innervated by efferent (sympathetic) and afferent nerves, but it is not clear whether these nerves affect cell formation or release in any significant way. To elucidate this problem, we studied mice neonatally sympathectomized with 6-hydroxydopamine and adult mice in which one hind limb was surgically denervated. Progenitor and transit cell numbers and proliferative activity were estimated in bone marrow, blood, and spleen. In addition, we performed unilateral electrical stimulation of nerve fibers to tibial marrow and applied a cell mobilizing stimulus (bleeding, granulocyte colony-stimulating factor injection, or intraperitoneal injection of a chemotactic substance) to investigate cell egress from the marrow. Blood flow to hindleg bone marrow was assessed with the radioactive microsphere technique. Except for a smaller bone marrow cell population and lower body weight in neonatally sympathectomized mice, we found no clear indications that bone marrow innervation influenced cell production. Also, the innervation did not detectably affect cell release from the marrow. Electrical stimulation of hind limb nerves did not change the blood flow to the marrow, whereas it markedly decreased blood flow to the overlying muscle. We therefore conclude that no obvious function can be ascribed to tibial marrow innervation in the mouse.
SYSTEMIC SIGNALS that have regulating influence on the bone marrow function, ie, formation and release of blood cells, are generally believed to be blood-borne (as reviewed by Benestad and Laerum1). However, a regulatory role has been suggested for the bone marrow innervation as well. For example, it has been suggested that the fine regulation of proliferation and release of hematopoietic cells is performed via nerve fibers that may be sensitive to minute pressure changes (pressure from hematopoietic growth or from blood flow), particularly in an organ that is protected from external pressure by a rigid bony capsule.2
It has been shown that afferent and efferent sympathetic nerves lie close to blood vessels within the marrow. Stromal and parenchymal cells are also innervated, although to a lesser extent than the vasculature.3-7 In addition to noradrenergic sympathetic nerve fibers, various peptidergic nerves to the bone marrow of rats and guinea pigs have been identified,8 in particular fibers containing substance P (SP) immunoreactivity.9 SP and neurokinin-A (NK-A), both coded by the preprotachykinin I gene, have been reported to release stimulatory and inhibitory hematopoietic cytokines from human bone marrow cells10-13 and blood monocytes.14 Furthermore, SP is a costimulant of various colony-stimulating factors10,15 and may even alone stimulate the formation of erythrocytic and granulocytic colonies.10 Finally, SP might affect the motility of granulocytes and monocytes16 and granulocyte infiltration into mouse skin17 and might thus possibly increase the rate of cell release from the bone marrow. Classical autonomic nerve transmitters might also modulate hematopoiesis in situ. Byron18 demonstrated that spleen colony-forming cells initiate DNA synthesis after stimulation with β-adrenergic or cholinergic agonists, and other workers19,20 have found a cholinergic enhancement of megakaryocytopoiesis and granulocytopoiesis in culture. On the other hand, Maestroni et al21 22 showed that chemical sympathectomy of adult mice and α1-adrenergic antagonists can enhance myelopoiesis and platelet formation.
Further evidence of a neuronal regulation of bone marrow function was provided by Foa,23 who claimed that electrical stimulation of the sympathetic chain or nerves sending branches to bone marrow led to vascular contraction and mobilization of erythrocytes, granulocytes, and immature cells into the blood of dogs. Similar findings have been made in rats, in which reticulocytes and granulocytes were mobilized by 1.5 to 2 hours of stimulation of both sympathetic trunks, with or without prior adrenalectomy or nephrectomy/ligation of renal vessels.24 25
Nevertheless, firm evidence for neuronal regulation of bone marrow function has to our knowledge not been presented. Therefore, we have used both well-established and new approaches to determine whether nerves can affect cell formation in or cell mobilization from bone marrow of the mouse. Mice were treated with 6-hydroxy-dopamine (6-OH-DA) shortly after birth to destroy the sympathetic nervous system. Some of these mice were adrenalectomized to remove this additional source of catecholamines, which might simulate sympathetic nerve transmitters. Hematopoiesis and marrow cell release were compared in these and sham-treated mice from the same litters. Furthermore, denervated or nerve-stimulated tibial marrow was sampled and compared with the contralateral control marrow in hormonally intact and in adrenalectomized mice. Steady-state and regenerating bone marrows were investigated. In the cell release studies, we examined whether nerve stimulation could mobilize cells to the blood or affect mobilization induced by injection of recombinant granulocyte colony-stimulating factor (G-CSF) or a chemotactic agent.
MATERIALS AND METHODS
Mice.
Female NMRI/Bom or ICR/OlaH mice were used. They were at least 4 and usually 7 to 15 weeks old and had free access to pellet food and tap water. A few experiments were performed with the youngest mice to find out whether nerves could play another role in a developing than in a mature hematopoietic system.
Except for the cell release experiments (see below), we performed surgery under midazolam (Dormicum; Roche, Basel, Switzerland) plus fentanyl/fluanisone (Hypnorm; Janssen, Beerse, Belgium) anesthesia and, except for the blood flow determinations (see below), the mice were killed with carbon dioxide gas.
Four main experimental designs were used: (1) cell generation in chemically sympathectomized mice; (2) cell generation in surgically denervated tibial marrow; (3) cell release in chemically sympathectomized mice; and (4) cell release from tibia during electrical nerve stimulation.
All experimental protocols were approved by the regional animal experimentation committee.
Chemical sympathectomy.
To destroy sympathetic neurons, mice received 6-OH-DA (Sigma Chemical Co, St Louis, MO) by subcutaneous injection (4 mg/mL in saline; 25 μL per gram of mouse weight; 4 injections; one every second day from when they were 2 days old)26 and otherwise treated as described.27 Control littermates received solvent injections.
Irides from 6-OH-DA– and solvent-treated mice were examined to establish the completeness of the chemical sympathectomy. They were stained with glyoxylic acid28 to ascertain that the denervation procedure had effectively removed all the catecholaminergic innervation. In only a few cases were insignificant remnants of nerve fibers found.
Surgical denervation.
One of the sciatic nerves was cut at the level of the sciatic notch. In initial experiments the femoral nerve was also cut just below the inguinal ligament. At the same time we performed a contralateral sham operation.
Smears made from marrow plugs, washed out of the tibiae with jets of medium, and small arteries sampled close to the tibial bones were stained with glyoxylic acid to check the denervation of the tibial marrow. Preparations from normal or sham operated legs showed a prominent plexus of fluorescent nerve fibers that was largely confined to the blood vessels (Fig 1). Two to 3 days after sciatic nerve section, this system of nerve fibers was either completely absent or reduced to occasional patches. This indicates that the sympathetic fibers to the tibial marrow are carried almost exclusively by the sciatic nerve (see also Weiss and Root29).
Sympathetic nerve fibers in tibial bone marrow. A dense plexus of nerve fibers with brightly stained varicosities is present around blood vessels. Digital image of normal bone marrow removed from mouse tibia and stained with glyoxylic acid. The diameter of the large vessel is about 30 μm. 40× water immersion objective.
Sympathetic nerve fibers in tibial bone marrow. A dense plexus of nerve fibers with brightly stained varicosities is present around blood vessels. Digital image of normal bone marrow removed from mouse tibia and stained with glyoxylic acid. The diameter of the large vessel is about 30 μm. 40× water immersion objective.
Electrical stimulation.
To study stimulation-induced cell release, mice were deeply anesthetized with chloral hydrate (0.2 g/kg) and pentobarbitone (0.05 g/kg) intraperitoneally (IP) and mechanically ventilated. An additional dose of the anesthetic mixture was supplied every 30 minutes. Both the left and the right sciatic nerves were cut, and the distal cut end of the right nerve was stimulated electrically. After a single test stimulus, further muscle contractions were eliminated by a neuromuscular blocking agent (1 mg/kg IP Alloferin; Hoffmann-LaRoche & Co AG, Basel, Switzerland). The nerve was then stimulated at 5 Hz continuously or 20 Hz for 5 seconds every 20 seconds for 50 minutes (so that the total number of stimulus pulses was 15,000 in both cases), with symmetrical bipolar current pulses (± 1 mA, 0.5 milliseconds each). The 5 Hz continuous stimulation was intended to release only adrenergic transmitters, whereas the 20 Hz pattern was intended to also release neuropeptides (see Lundberg et al30). The animals were then killed with neck luxation and tissues were removed immediately for analysis.
Adrenalectomy.
Some mice were delivered adrenalectomized or sham adrenalectomized from the vendor (Gml. Bomholtgaard, Ry, Denmark) or the national importer of experimental animals (SIFF, Oslo, Norway). These procedures were later performed in our own laboratory.27 These mice drank 0.9% saline. The adrenalectomy had been performed at least 4 weeks before the cell generation experiments and at least 4 days before the cell release experiments.
To monitor the completeness of the adrenalectomies, corticosterone (the major adrenal glucocorticoid hormone in the mouse) in slightly diluted mouse heparin plasma was analyzed after ether extraction of 100 μL plasma with a previously described radioimmunoassay.27 The intra-assay coefficient of variation is between 6% and 10%. In 8 hormonally intact mice, the quartile interval for the corticosterone concentration was 350 to 570 nmol/L (for the plasma protein concentration, 28 to 32 mg/mL). The corresponding data for 23 adrenalectomized mice were 80 to 170 nmol/L and 28 to 35 mg/mL, respectively. Three mice in this group had values indicating incomplete removal of the adrenals, which did not significantly affect the median values.
Bone marrow, spleen, and blood examinations.
The surgically denervated tibial bones or the femurs from chemically sympathectomized mice were dissected free and crushed in a mortar with cold serum-containing culture medium. The marrow cells were suspended by vigorous pipetting, counted with a Coulter counter, smeared, stained, and classified microscopically (200 cells counted from each mouse, the identity of the preparation being unknown to the examiner). Megakaryocytes were scored after acetylcholine esterase staining.
The differential counting of hemoglobin-containing nucleated cells was facilitated by prestaining the smears with o-dianisidine.31
Reticulocytes were stained with Brilliant cresyl blue and 500 to 1,000 cells were scored from each sample of mouse blood. Total and differential blood cell counts (100 to 200 cells per mouse) were performed as for the marrow. Alternatively, reticulocyte and differential counting was performed with automatic cell counters (R-1000 Sysmec [Kobe, Japan] and Technicon H-1 [Tarrytown, NY]).
Standard methods were used to make single-cell suspensions of spleen cells, to determine the packed blood cell volumes (hematocrit), and to analyze plasma protein concentrations.
Regenerating marrow cells were obtained 3 days after injection of 200 mg/kg IP cyclophosphamide (Sendoxan; Asta Medica, Frankfurt, Germany).
We enumerated granulocyte-macrophage colony-forming cells (G/M-CFC = GM-CFC, G-CFC, and M-CFC) by culturing 5 × 104steady-state or 2 × 104 regenerating marrow cells and 0.5 × 106 or 1 × 106 spleen cells in methylcellulose-containing medium (1 mL) for 7 days with pokeweed mitogen-stimulated spleen cell conditioned medium as a source of colony-stimulating factors. Cell aggregates with more than approximately 50 cells were scored as colonies.32
As an alternative to the standard colony scoring method, dishes were scored automatically with a digital image analysis system applied to video pictures of the dishes.33 Viable cells in the cultures had then been stained supravitally beforehand.34
Flow cytometry of single-cell suspensions of bone marrow was performed with a FACScan (Becton Dickinson, Mountain View, CA), the unstained cells (10,000 cells per sample) being classified according to their light forward and side scatter properties. The positions of mature granulocytes, small lymphocyte-like cells, and macrophage-like cells in the dot plots thus obtained had been determined in pilot experiments with less heterogeneous cell populations. These were peritoneal cells harvested at different intervals after injection of a chemotactic agent (Bacto-Tryptone; see below) and subjected to both flow cytometry and standard differential counting.
Bone marrow proliferative activity was assessed with measurements of3H-thymidine incorporation 1 hour after the intravenous (IV) injection of 37 kBq 3H-thymidine/g body weight (code TRK 120; Amersham, Amersham, UK) in approximately 0.2 mL medium. Radioactivity in trichloroacetic acid cellular precipitates and in a diluted sample of the 3H-thymidine prepared for injection was counted in a β-counter.
Granulocyte mobilizing agents.
Bacto-Tryptone (10% in saline, 1 mL per mouse; Difco Labs, Detroit, MI), which is a caseı̈n digest, was used to mobilize bone marrow neutrophils, because it has a strong, chemotactic effect after IP injection and also markedly increases the concentration of these cells in the blood.35 Human recombinant G-CSF (5 μg/kg IV; Chugai Pharmaceutical Co, Ltd, Tokyo, Japan) was also used to release marrow neutrophils.36
To assess the potency of G-CSF to mobilize PMN to blood, we sampled twice from a cut metatarsal vein; otherwise, blood was withdrawn from the inferior caval vein of the killed mice.
Blood flow measurements.
We used the radioactive microsphere method to determine organ perfusion in anesthetized mice. This method has been thoroughly described by Iversen et al,37 as used in the present study and also validated for the mouse.38 Briefly, about 40,000 well-mixed microspheres (diameter, 16 μm; NEN, Boston, MA) labeled with either153Gd or 51Cr were injected as a bolus into the left ventricle after the chest had been opened in the midline. The two femurs, tibiae, and the two soleus muscles were removed, as well as the two kidneys. The radioactivity of these samples was determined in an Auto-Gamma 5220 counter (Packard, Downers Grove, IL). The lowest number of microspheres deposited in any sample exceeded 250.
Statistics.
The values are expressed as medians with or without their corresponding 95% confidence intervals, determined with a nonparametric method (program MINITAB; Minitab Inc, State College, PA). If the median of a control group was not included in the 95% confidence interval of the test group, and vice versa, then the two groups of data were considered significantly different at the 5% level. With only one of the group medians falling outside the other group's interval, the two-sided significance was tested with Wilcoxon-Mann-Whitney two-sample test (MINITAB). When appropriate, Wilcoxon's test for paired comparisons or the nonparametric Wilcoxon-van Elteren test for paired groups of data39 was applied.
RESULTS
Bone marrow hematopoiesis.
To examine the effect of the sympathetic innervation on hematopoiesis, we studied mice that had been chemically sympathectomized by 6-OH-DA shortly after birth. We found no significant influence of this treatment on blood cell concentrations (Fig2). However, the bone marrow cellularity was lower in 6-OH-DA mice than in the controls, whereas the 3H-thymidine incorporation per femur and the differential counts were apparently normal (Fig 3). No differences were detected between tests and controls concerning the concentrations of granulocyte and macrophage colony-forming cells, either in steady-state or in regenerating bone marrow and spleen (Table1). However, the cellularities of marrow and spleen were lower in the sympathectomized mice 3 days after cyclophosphamide had been administered to kill cycling cells and provoke a regenerative response (Table 1). The body weights were also lowest in the experimental group (95% confidence intervals of the medians: males, 33 to 39 g v37 to 42 g; females, 26 to 30 g v 27 to 32 g;P = .001 for all litters analyzed according to treatment group and gender).
Blood cell concentrations in chemically sympathectomized and control animals. Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.
Blood cell concentrations in chemically sympathectomized and control animals. Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.
Decreased femoral marrow cellularity, but no change in proliferative activity and differential counts in chemically sympathectomized mice. The mice were killed 2 hours after Bacto-Tryptone injection. *P = .002. (For these data pooled with data from another series of experiments [17 + 19 cyclophosphamide-treated mice], P < .0001). Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.
Decreased femoral marrow cellularity, but no change in proliferative activity and differential counts in chemically sympathectomized mice. The mice were killed 2 hours after Bacto-Tryptone injection. *P = .002. (For these data pooled with data from another series of experiments [17 + 19 cyclophosphamide-treated mice], P < .0001). Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.
We then let each experimental animal serve as its own control by surgically denervating one hind limb. Paired comparisons did not show significant differences between the denervated and the intact tibia. This applies to numbers of both progenitors (G/M-CFC) and more mature cells, 2 or 7 days after one-sided denervation of normal as well as adrenalectomized mice (Table 2). Similarly, experiments with adrenalectomized or hormonally intact mice did not disclose any consistent effects of denervation on regenerating marrow examined 3 to 6 days after the injection of cyclophosphamide (eg, for all days taken together, the 95% confidence interval for the difference in cellularity between innervated and denervated tibiae was [−1.1, 0.1] × 106 cells, P = .08, n = 29 mice). A representative experiment is shown in Fig 4. The regeneration model had been validated in experiments where we measured tibial marrow cellularities before and 3, 5, and 6 days after cyclophosphamide. Rapid cell proliferation took place, with significant cellularity increases between successive days of regeneration and between day 6 and the steady-state level (data not shown).
Bone marrow regeneration after cyclophosphamide treatment. Eight mice had one leg denervated and the other sham operated; thereafter, they received cyclophosphamide at 200 mg/kg IP (day 0). From day 3 on, 2 mice were killed at each time point and cellularities compared between innervated and denervated tibia. The stippled belt depicts the 95% confidence interval for total tibial cell number in untreated mice. Digital image analysis of the methylcellulose dishes gave two sets of data in addition to the colony numbers (G/M-CFC/tibia), ie, (1) the total cellularity per dish when 5 × 104 cells had been cultured for 7 days (ie, G/M cytopoietic capacity per tibia in arbitrary units [au]) and (2) median colony size. (•) Innervated; (○) denervated.
Bone marrow regeneration after cyclophosphamide treatment. Eight mice had one leg denervated and the other sham operated; thereafter, they received cyclophosphamide at 200 mg/kg IP (day 0). From day 3 on, 2 mice were killed at each time point and cellularities compared between innervated and denervated tibia. The stippled belt depicts the 95% confidence interval for total tibial cell number in untreated mice. Digital image analysis of the methylcellulose dishes gave two sets of data in addition to the colony numbers (G/M-CFC/tibia), ie, (1) the total cellularity per dish when 5 × 104 cells had been cultured for 7 days (ie, G/M cytopoietic capacity per tibia in arbitrary units [au]) and (2) median colony size. (•) Innervated; (○) denervated.
The preparatory operations per se did not affect the hematopoietic system as far as we could detect by enumerating blood, spleen, and bone marrow cells 2 and 4 days after sham dener- vation and at least 4 weeks after adrenalectomy (data not shown).
Cell release from bone marrow.
We counted neutrophilic granulocytes (PMN) and reticulocytes. PMN is the only mature cell type with a large storage compartment in the bone marrow, and reticulocytes may also be rapidly released (mobilized). Various humoral stimuli can release cells from the bone marrow. Our mobilizing agent was Bacto-Tryptone injected IP 2 hours before cell sampling in combination with bleeding approximately 10% of estimated blood volume.
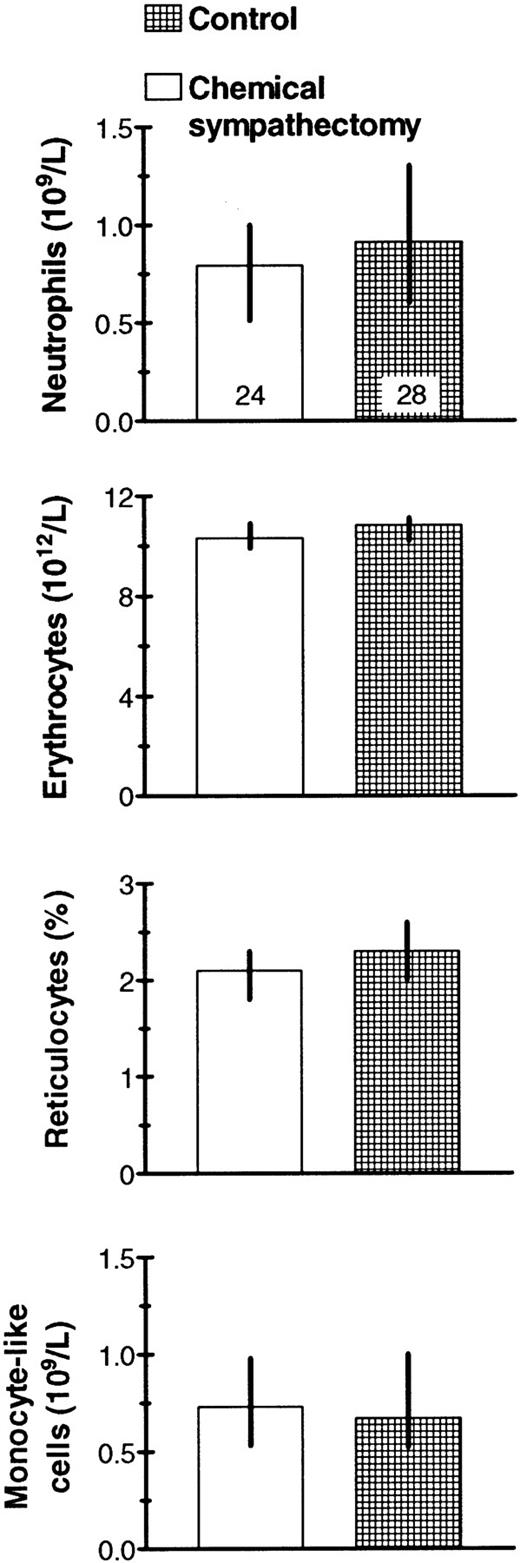
Neither the numbers of PMN in the peritoneal cavity nor the concentration of reticulocytes in the blood was detectably different in chemically sympathectomized mice and intact littermate mice (Fig 5). Moreover, we did not find significantly different sizes of the neutrophil storage compartments in the bone marrow after cell release in the two groups of mice (data not shown).
No significant effects on peritoneal and blood cell populations of Bacto-Tryptone IP and slight (0.2 to 0.3 mL) bleeding. Blood reticulocyte change refers to reticulocyte percentages in venous blood 2 hours after and just before the IP Bacto-Tryptone injection. Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.
No significant effects on peritoneal and blood cell populations of Bacto-Tryptone IP and slight (0.2 to 0.3 mL) bleeding. Blood reticulocyte change refers to reticulocyte percentages in venous blood 2 hours after and just before the IP Bacto-Tryptone injection. Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.
Similarly, we could not detect any significantly different neutrophil storage pool in the denervated compared with the innervated tibial marrow in mice treated with Bacto-Tryptone (Table 3).
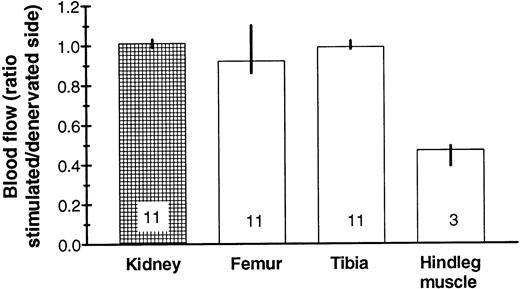
In further experiments, the sciatic nerve was cut bilaterally and the distal cut end was stimulated electrically for about 50 minutes on one side. A mobilizing stimulus (Bacto-Tryptone IP or G-CSF IV) was applied here in some of the experiments 3 to 4 hours beforehand. Again, we could not detect any systematic differences in mature neutrophil numbers between the two tibiae (95% confidence intervals for the differences: [−1.17, 0.02] × 106 cells with mobilization and [−1.62, 0.55] × 106 cells without; the positive control being the presence of a blood granulocytosis after G-CSF treatment). Moreover, the nerves seemed to have no influence on marrow blood flow, as expected,37whereas a markedly different blood flow to the musculature of the two legs was found (Fig 6).
No significant difference in blood flow was found between denervated and stimulated tibial marrow, in contrast to overlying muscle. The ratio is between the right and left side in mice with denervated left and (neurally) stimulated right hindleg. Median values are given with their 95% confidence intervals (except for the muscle, in which the range is depicted) as well as the number of mice examined.
No significant difference in blood flow was found between denervated and stimulated tibial marrow, in contrast to overlying muscle. The ratio is between the right and left side in mice with denervated left and (neurally) stimulated right hindleg. Median values are given with their 95% confidence intervals (except for the muscle, in which the range is depicted) as well as the number of mice examined.
DISCUSSION
Except for a modestly decreased total number of cells in the bone marrow of neonatally sympathectomized mice, we found no conspicuous nerve effects on cell generation in, or mobilization from, the marrow. Our results are consistent with those from previous rat experiments, where bone marrow nerves did not affect the energy metabolism of marrow cells.40
A convincing demonstration of the roles of bone marrow nerves should establish a relationship between nerve activity and cell formation in or cell egress from the marrow. However, detailed, putative mechanisms have been clarified, mostly in vitro, whereas the physiologic evidence is still scant. For example, spleen colony-forming stem cells initiate DNA synthesis in vitro after stimulation with β-adrenergic agonists,18 and human hematopoietic cells can be stimulated by SP in vitro, apparently both directly and indirectly via stromal cell release of cytokines.10-12 An elaborate, bidirectional interplay between bone marrow nerves on the one hand and stroma and mononuclear cells on the other might exist, but has not been directly shown, in which SP and cytokines through transcriptional mechanisms might influence each other, both concerning secretion rates and receptor expression. Therefore, although nervous regulation is generally used for rapid adaptations (within a time frame of seconds, and cell generation and mobilization from the marrow are outside this range), it is plausible but not substantiated that the nerves to bone marrow may play a regulatory role.
Indeed, a rich nervous network has been found in the marrow, consisting of afferent fibers, mostly myelinated (4%), and efferent, sympathetic fibers that are unmyelinated (96%), as reviewed by Yamazaki and Allen.6 These investigators found that most efferent fibers end close to marrow arterioles, but rarely in hematopoietic parenchyma (5.3% of the fibers) or on venous sinus walls (2.7%). Their electron microscopy and morphometric studies also showed what might be a functional unit for signal conduction (the neuro-reticular complex) composed of efferent nerves apparently synapsing with stromal cells that are connected by gap junctions.
DePace and Webber25 described adrenergic nerves in rat bone marrow, and Felten et al9 claimed that noradrenergic postganglionic sympathetic nerve fibers innervate bone marrow and that SP-immunoreactive fibers had been observed in some sections of bone marrow. Weihe et al8 stated that, despite unsatisfactory staining quality, they had observed nerve fibers containing calcitonin gene-related peptide- and tachykinin-immunoreactivity in vascular and nonvascular locations of rat and guinea pig bone marrow. Some fibers with immunoreactivity to tyrosine hydroxylase and neuropeptide Y were also seen. However, it should be noted that non-nervous sources of the most studied neuropeptide, SP, have been described, namely macrophages41,42 and endothelial cells,43 44with both cell types being constituents of bone marrow stroma.
The picture is further complicated by the recent finding that NK-A, formed by alternative splicing of the messenger of the preprotachykinin 1 gene (which also codes for SP), stimulates erythroid progenitors, but inhibits proliferation of GM-CFC, partly through stromal secretion of the inhibitory cytokines transforming growth factor-β and macrophage inflammatory protein-1α.13 A neural fine-tuning of hematopoiesis, based on these stimulatory and inhibitory SP and NK-A mechanisms, might be consistent with our own finding that denervation did not affect hematopoiesis, except that both SP and NK-A apparently stimulated erythropoiesis in vitro.10-12
Maestroni et al21,22 found that adrenergic agents could inhibit myelopoiesis and platelet formation in mice, both under steady-state conditions and after irradiation and syngeneic marrow transplantation. Based on experiments with chemically sympathectomized adult mice (by 6-OH-DA) and the α1-adrenergic antagonist prazosin, they stated that the production of granulocytes and macrophages seems to be under an inhibitory noradrenergic tone. Our results do not support this view. It is possible that effects, which may be both nonspecific and not related to the innervation of bone marrow, may take place after treatment of adult, nonadrenalectomized animals with 6-OH-DA. The same kind of objection could be raised against the interpretation of the prazosin results. On the other hand, it could be argued that we had missed important clues by working with neonatally sympathectomized mice. Thus, neonatally sympathectomized rats had adapted to the small immune deviations observed shortly after the 6-OH-DA treatment, when they were 42 to 56 days old, possibly due to partial reinnervation of lymphoid organs and compensation by the adrenal glands and other hormonal systems.45 However, nerve regeneration was usually not detected in our experiments (iris examinations; see the Materials and Methods), and we could not find qualitatively different effects of sympathectomy or surgical denervation between mice with and without adrenal glands.
Direct neural evidence for regulation of bone marrow function (obtained by denervation, nerve stimulation, or measurement of nerve activity) has, to our knowledge, been reported from only two laboratories. Foa23 worked with anesthetized dogs, in which he cut the distal tibia and recorded expansion and contraction of the tibial marrow. Moreover, venous blood from the marrow was sampled. The sciatic nerve and the lumbar sympathetic chain were cut and the distal ends were stimulated. An immediate reduction in volume of the marrow was found (see also Weiss and Root29). After the electrical stimulation, the venous effluent from the marrow contained an increased number of white and red blood cells, including immature cells. Because the tibial marrow of young dogs is not very active hematopoietically, experiments were also performed on rib marrow, with stimulation of intercostal nerves and blood sampling from an intercostal vein. Here too, the cell concentrations increased during the stimulation, especially concerning the immature cells. However, there were no sham-stimulated controls, no endotoxin or corticosteroid analyses, and no plausible explanation of the mobilization of immature cells, which are very scarce in the blood under normal physiologic circumstances.
Webber et al24,25 stimulated the sympathetic trunk bilaterally in albino rats and observed release of reticulocytes and neutrophils into the blood. In some rats, adrenalectomy and nephrectomy were performed before the stimulation. However, absolute cell counts were not performed—only differentials. It is puzzling that no changes were recorded in the controls; after all, they had been exposed to laparotomy and some of them also to operations on the distal femurs (a hole was drilled to aspirate marrow before the electrical stimulation). A shift of lymphocytes to bone marrow46 and mobilization of neutrophils to blood (as part of a corticoid stress response; reviewed by Benestad Laerum1) might have explained their findings. Moreover, based on data published by others,47-49 the decline in marrow neutrophil percentage (loss of about 10% of all marrow cells) should have led to a much more marked increase in blood neutrophils than was actually found (about 28%) if the only relevant perturbation of homeostasis in these rats was the stimulated marrow cell egress. We have tried to reproduce the findings of Webber et al. No significant reticulocytosis was observed, and no consistently different blood PMN response between the lumbar sympathetic trunk-stimulated and sham-stimulated rats (our unpublished observations) was observed either.
It has been reported, based on perfusion experiments, that increased cell release accompanies increased blood flow through the bone marrow.50 We hypothesized that perturbation of the sympathetic innervation of marrow blood vessels would lead to a change in blood flow and hence a change in cell release rate. However, even though the vasculature in the overlying muscle was sensitive to the nerve stimulation, the marrow vasculature proved nonresponsive. This is in accord with our previous findings in the rat with both surgically37,51 and chemically52sympathectomized animals. Similarly, stimulation of the sympathetic trunk in the rat decreased blood flow to hind limb skin and muscle, but not to the bone marrow (our unpublished observations). Even though intricate nerve arrangements have been described in the bone marrow (see above), sympathetic innervation may be without physiologic significance, at least in the mouse (but possibly not in other species, see Drinker and Drinker53). Conceivably, the innervation may play a role under special, but unknown, conditions. For example, studies that have used radioactive microspheres to measure cerebral blood flow have shown that neural effects are minimal, except during extreme hypertension.54 The role of the vasoconstrictor innervation may here be to protect the blood-brain barrier against disruption should arterial pressure increase suddenly. This teleologic explanation may not be valid for the bone marrow; moreover, the generally accepted regulatory mechanisms for hematopoiesis and cell release from the marrow are all humoral, namely endocrine, paracrine, or autocrine.1 55
Our conclusion that tibial bone marrow innervation in the mouse does not influence cell production or cell release (or at least not directly, in a biologically significant way, and under ordinary circumstances) supports the physiologic relevance of ex vivo experiments on bone marrow.
ACKNOWLEDGMENT
The authors are indebted to Prof K. Hirashima and Dr N. Sato for the generous gift of recombinant human G-CSF, produced by Chugai Pharmaceutical Co (Tokyo, Japan). We also thank Dr Liv Theodorsen, MD (The Central Laboratory, The Norwegian Radium Hospital, Oslo, Norway) for letting us examine mouse blood with the blood cell analyzers.
Supported by grants from the Norwegian Research Council and from Anders Jahre's Foundation for the Promotion of Science.
Address reprint requests to Haakon B. Benestad, MD, Department of Physiology, Institute of Basic Medical Sciences, University of Oslo, PO Box 1103 Blindern, N-0317 Oslo, Norway.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 3. Decreased femoral marrow cellularity, but no change in proliferative activity and differential counts in chemically sympathectomized mice. The mice were killed 2 hours after Bacto-Tryptone injection. *P = .002. (For these data pooled with data from another series of experiments [17 + 19 cyclophosphamide-treated mice], P < .0001). Median values with their 95% confidence intervals and the number of mice examined (top columns) are given.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1280/4/m_blod4041103.jpeg?Expires=1765019615&Signature=t4BLmqbg6~FIkL-axNWy8i76tWLh7qhzTvvpGHre0l-GpuGr1wI-RJCVj1Jstm1~IQpaGS~QLWnstSOzJgN3MdtpbGT3tTTvghDIA1yBgNzi7GtGAi6y0g-BRZw2YVf0eo6glUwMDO7ynfnfEYKpOtORVAXLiz2qd3RSFh590WHsdY9cfz3fAjPuN5RvySn2xxlG1tVj7X92ZYJsHI3x5LtVHpmBovhYuchyP9hSjuG-KgSYPKzv-Ex6rLWoTTzn19MCJCDY6B5SW4Nw-q6~Hwqa8C~15wNBrzmJJFcGEfg7VXFrjNwM7w1ofMya5359e6JoxsZ1kbZ9PRJKUQUamA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Bone marrow regeneration after cyclophosphamide treatment. Eight mice had one leg denervated and the other sham operated; thereafter, they received cyclophosphamide at 200 mg/kg IP (day 0). From day 3 on, 2 mice were killed at each time point and cellularities compared between innervated and denervated tibia. The stippled belt depicts the 95% confidence interval for total tibial cell number in untreated mice. Digital image analysis of the methylcellulose dishes gave two sets of data in addition to the colony numbers (G/M-CFC/tibia), ie, (1) the total cellularity per dish when 5 × 104 cells had been cultured for 7 days (ie, G/M cytopoietic capacity per tibia in arbitrary units [au]) and (2) median colony size. (•) Innervated; (○) denervated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1280/4/m_blod4041104.jpeg?Expires=1765019615&Signature=eNpKWRQFuHt6P2mSGyOhWqZc-zjdbyMAWN0TXIqaIAQ5tQ0QygbfWfvJ~2uAtVSkZhRsapa4leExWJQkEUEgWQR-H9PhTgST9iU1cxRVKKl4U-172x8~sbsJcVH4iOHKDaCIOirr8WEiJTCIyRB~WT3e0f-C5JFAMlqOsmQK43ZjTxy3iGDF2fbWFDKP6UHjphgkVKjtPU09Bl2DiASNmKjFW~HyR7xR0QLNN8JZMi-IU-imp0gGu1IxfxO5Bj8c~VjZVpuvA41n3fNPa3-B-TN1v49QebleM2M7sPGhX7rPvfVbEbDJmJph2XAqVHBHmp8Z-WDcu-BwE3bJlyL4yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)