Abstract
To assess the risk factors, natural history, and eligibility for curative treatment of early-detected hepatocellular carcinoma (HCC), 385 hemophiliacs who were treated with blood or plasma derivates for at least 10 years and had persistently elevated aminotransferase values underwent an annual screening with an abdominal ultrasound examination and measurement of the serum alpha-fetoprotein (AFP) level. Of these, 355 had serum antibody to hepatitis C virus (anti-HCV), 29 had anti-HCV and hepatitis B surface antigen (HBsAg), and one had HBsAg alone; 141 had serum antibody to human immunodeficiency virus (anti-HIV). During 48 months of follow-up study, six patients developed HCC. All HCC patients had a HCV-related cirrhosis and had been exposed to HCV risk at a median age of 40 years. All patients had a multicentric tumor, which was not eligible for curative treatment. Univariate analysis showed age, cirrhosis, and baseline AFP levels to be significantly associated with an increased risk of HCC. By multivariate analysis, the risk of HCC was infinite in patients with cirrhosis, 31.0 for those with baseline AFP higher than 11 ng/mL, and 17.9 for those more than 45 years of age. In conclusion, the risk of cancer was greater for patients infected later in life, particularly those with cirrhosis and high AFP. Annual screening of hemophiliacs with ultrasound and AFP fails to identify potentially curable tumors because the diagnosis is made at a late stage of the disease.
SEQUENTIAL DEVELOPMENT of cirrhosis and hepatocellular carcinoma (HCC) has been documented in nonhemophilic patients with chronic liver disease due to hepatitis C virus (HCV) or hepatitis B virus (HBV).1,2 Preliminary evidence suggests that this is also true for hemophilic patients who acquired chronic viral hepatitis subsequent to replacement therapy with large-pool clotting factor concentrates infected with blood-borne hepatitis viruses.3-5
In a questionnaire-based survey of 11,801 multitransfused hemophiliacs from 54 centers in the United States and Europe, 10 cases of HCC were reported that were invariably associated with cirrhosis due to either HBV or HCV.4 In the survey, the crude rate of HCC in hemophiliacs was 30 times the background incidence of the tumor in the countries of origin of the patients, and in most instances the tumors were so advanced they could not be successfully treated with surgery. The only exceptions were three patients, two of whom had undergone studies with abdominal ultrasound examination and/or measurement of serum markers of HCC such as alpha-fetoprotein (AFP), in whom HCC was less advanced and could be treated with chemotherapy or surgery.4 These findings suggest that screening may enhance the chance of early HCC detection in patients with chronic viral hepatitis, although it remains unclear whether survival is improved by early diagnosis.2 6-8
Multitransfused hemophiliacs offer a unique model for studying the sequelae of chronic hepatitis C. The duration of hepatitis risk can be calculated virtually in all patients: most of the older patients have had exposure to blood, plasma, or cryoprecipitates that had a high risk of transmitting HCV.9 In the 1970s, practically all patients treated with non–virus-inactivated concentrates became infected with HCV after their first infusion of concentrates prepared from a large plasma pool.10 Another peculiar feature of HCV infection in hemophiliacs is that the course of hepatitis may be aggravated by multiple virus infections and impaired immunity as a consequence of repeated infusions with infected concentrates.11 12 To assess the natural history of HCC and whether screening enhances early detection and improves the chances of curative treatment, we began in 1992 to prospectively study 385 multitransfused hemophiliacs with a long history of elevated aminotransferases (ALT) using an annual abdominal ultrasound examination and serum AFP measurement.
MATERIALS AND METHODS
Participating centers.
In January 1992, all hemophilia centers in Italy were invited to participate in a prospective study aimed at the early diagnosis of HCC through an annual screening program. Eleven Centers agreed, six in Northern Italy, one in Central Italy, and four in Southern Italy.
Enrollment and follow-up study.
Patients were enrolled if they had been treated with large-pool clotting factor concentrates for at least 10 years before the start of the study and had serum aminotransferase activity greater than 1.5 times the upper limit of normal at three consecutive tests 6 months apart. At enrollment, all patients underwent a full clinical examination and routine liver chemistry analysis. Patients were also tested for serum markers of HBV and HCV (hepatitis B surface antigen [HBsAg] and anti-HCV, second-generation ELISA; Ortho Diagnostics, Raritan, NJ) anti-HIV (ELISA; Ortho), and AFP (ELISA; Abbott Laboratories, Chicago, IL). Abdominal ultrasound examination was performed using conventional real-time equipment. Every 6 months, all patients repeated the clinical examination and routine liver chemistry analysis. Every 12 months, patients were also studied with abdominal ultrasound examination and serum AFP measurement.
Cirrhosis was established on the basis of clinical signs of portal hypertension (platelet count <100,000/μL, albumin level <3.5 g/L, and serum cholinesterase activity <4.5 U/L), endoscopic signs (esophageal varices and portal hypertensive gastropathy), and/or abdominal ultrasound examination (irregular margins of the liver, dilated portal vein axis, and splenomegaly). The clinical stage of the disease was classified according to the Child-Pugh score13: 1, bilirubin less than 2 mg/100 mL, albumin greater than 3.5 g/dL, prothrombin time 2 to 4 seconds longer than normal, and no ascites or encephalopathy; 2, bilirubin between 2 and 3 mg, albumin between 2.8 and 3.5 g, prothrombin time 4 to 6 seconds longer, first- to second-degree encephalopathy, and modest ascites; 3, bilirubin greater than 3 mg, albumin less than 2.8 g, prothrombin time at least 6 seconds longer than normal, third- to fourthdegree encephalopathy, and severe ascites; 5 to 6, Child-Pugh class A; 7 to 9, Child-Pugh class B; and greater than 9, Child-Pugh class C.
Diagnosis of HCC.
HCC was diagnosed on the basis of abdominal ultrasound identification of a focal lesion in a patient with serum AFP higher than 400 ng/mL. Patients with a focal lesion on abdominal ultrasound and serum AFP less than 400 ng/mL were considered suspect for HCC and underwent a diagnostic liver biopsy with an echo-guided thin needle (21-gauge Tru-cut needle; Travenol, Hyland, Los Angeles, CA).
Tumor staging.
To assess the number of tumor nodes, tumor size, and extrahepatic metastasis, patients with potentially operable HCC were further investigated with chest x-ray, abdominal computed tomographic (CT) scan, bone scintiscan, and Lipiodol hepatic arteriography (Lipidol Ultrafluide, Laboratoire Guerbet, Aulnay-Sous-Bois, France). The disease was classified as unicentric when a single tumor node was detected with ultrasound and confirmed with CT after Lipiodol hepatic arteriography. It was considered multicentric when more than one node was detected with abdominal ultrasound and/or other imaging techniques. To calculate tumor size, the two main diameters of the lesion(s) were measured by CT scan.
Treatment options.
The following therapeutic algorithm was developed. Orthotopic liver transplantation was thought to be the best option for an anti-HIV–seronegative patient who had cirrhosis, was younger than 60 years, and had a single tumor less than 3 cm in diameter. Patients who did not have cirrhosis or did not fit the general criteria for transplantation were considered for hepatic resection or transhepatic arterial chemoembolization with doxorubicin. Patients who were not eligible for any of these treatments were given palliative treatment such as analgesics, antipyretics, or corticosteroids if required.
Statistical analysis.
Student's t test was used to compare means and the chi-square test to compare proportions. Unconditional logistic regression was used to calculate the odds ratio (OR) of HCC according to age, AFP level, and presence of cirrhosis. Survival estimates from birth to the age at development of HCC were calculated by Kaplan-Meier curves and compared by the Wilcoxon test and log-rank test. Conditional risk ratios were calculated using the proportion hazard regression (PHREG) procedures, taking into consideration the following variables: sex, AFP more than 11 ng/mL (95th percentile of the control distribution), duration of follow-up study (<90, 190 to 240, or >240 months), and type of hemophilia.
RESULTS
Of 1,675 patients attending the 11 participating centers, 385 (22.9%) met the inclusion criteria. There were 377 males and eight females aged 10 to 83 years (median, 31); 311 patients had hemophilia A, 60 had hemophilia B, and 14 had von Willebrand disease. Patients had been treated with blood, plasma, cryoprecipitates, or clotting factor concentrates for a median of 240 months (range, 120 to 588); 355 patients had serum anti-HCV alone, 29 had both serum anti-HCV and HBsAg, and one had HBsAg alone; 141 (36.6%) patients had serum anti-HIV; and 40 patients (10.3%) had cirrhosis (Table1). Eleven patients had Child-Pugh A cirrhosis, nine Child-Pugh B, and 20 Child-Pugh C. The prevalence of cirrhosis was similar in anti-HIV–positive and anti-HIV–negative patients (13.4% v 8.6%, P = .09). During a 48-month follow-up study, 36 patients (9.3%) died. Twenty died of HIV-related events; six anti-HIV–positive patients died of liver failure. Among the anti-HIV–negative patients, nine, including four with HCC, died of liver failure and one died of causes unrelated to the liver. Overall, 15 hemophiliacs (3.9%) died of liver-related disease.
Six patients developed HCC, with an incidence rate of 390 cases per 100,000 per year. Table 2 summarizes the main epidemiologic and clinical features of the six patients with HCC. The median age at which these patients were first exposed to blood or plasma concentrates was 40 years (range, 22 to 50), compared with 12 years (range, 0 to 53) for the other patients (P < .0001). In all cases, HCC was associated with HCV-related cirrhosis due to infection with genotype 3a in two, 3a and 1a in one, 1a in one, and 1b in another. For one patient, no serum sample was available for genotyping. In all cases, the tumor was multicentric: when the tumor was first detected, two patients had two nodes and four had more than three. At diagnosis, serum AFP was higher than 400 ng/mL only in one patient (case no. 1) and was above the upper-normal limit (7 ng/mL) but less than 400 ng in five patients (no. 2 to 6). Liver function was good in three patients (no. 3, 5, and 6), but was impaired in the remaining three. None of the patients met the criteria for surgery or treatment with arterial chemoembolization. One patient was unsuccessfully treated with intravenous mitoxantrone. The survival time from diagnosis to death was 4 to 19 months (Table 2).
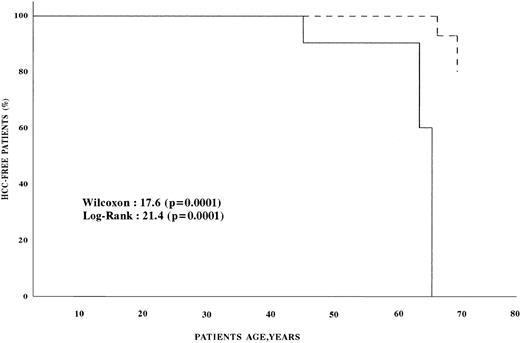
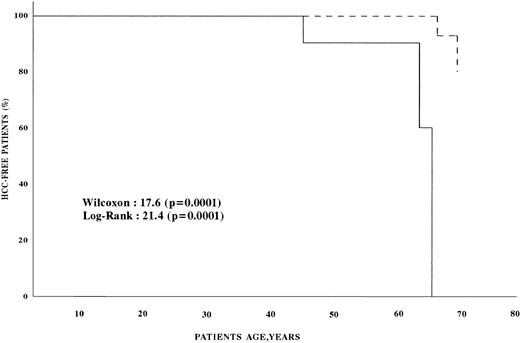
Univariate analysis found that the age, presence of cirrhosis, and AFP level at enrollment were significantly associated with an increased risk of HCC (Table 3). Multivariate analysis showed that the risk of HCC was infinite in patients with cirrhosis, 31.0 (confidence interval [CI], 4.8 to 199.2) in patients with AFP more than 11 ng/mL, and 17.9 (CI, 1.9 to 173.3) in patients older than 45 (Table 4). Figure 1 shows the survival estimates from birth to the age of development of HCC for patients with AFP less than 11 ng/mL at baseline and for those with higher baseline AFP. Comparative analysis of the two curves shows that HCC developed earlier in patients with AFP greater than 11 ng/mL (P < .0001). The conditional risk ratio of HCC in patients with baseline AFP higher than 11 ng/mL was 70.4 (CI, 3.6 to 1,374.7).
HCC-free survival of hemophilic patients. (---) Survival of patients with serum AFP <11 ng/mL; (—) survival of patients with serum AFP >11 ng/mL.
HCC-free survival of hemophilic patients. (---) Survival of patients with serum AFP <11 ng/mL; (—) survival of patients with serum AFP >11 ng/mL.
DISCUSSION
This prospective study confirms previous retrospective surveys4 5 showing that HCC is an important cause of death in both anti-HIV–negative and anti-HIV–positive hemophiliacs with chronic hepatitis C. The risk of liver cancer is particularly high in hemophiliacs with cirrhosis and elevated AFP, being higher in patients infected late in life than in those infected earlier.
The yearly HCC incidence rate of 390 cases per 100,000 in our patients is much higher than the calculated yearly age-adjusted incidence of HCC in the general population in Italy, which is approximately seven cases per 100,000.14 The incidence rate of HCC in these prospectively studied hemophiliacs was also higher than in our previous retrospective study.4 That study enrolled all unselected patients with hemophilia, whereas this prospective study focused on patients with chronic ALT abnormalities, who have a high risk of cirrhosis and HCC.15 16 All six patients with HCC had clinical or histologic features of cirrhosis due to chronic infection with HCV.
Persistent hepatocellular inflammation is a well-recognized triggering factor for HCC in patients with chronic hepatitis C.1,2,16Every year, 3% to 6% of prospectively evaluated patients with HCV-related cirrhosis develop liver cancer.1,2,8,16,17Thus, the low annual rate (0.4%) of HCC detected in our hemophiliacs might simply reflect the small proportion of patients with cirrhosis enrolled. Although the lack of histologic studies could underestimate the real prevalence of cirrhosis in hemophiliacs, our finding of 10% of the patients showing clinical or ultrasound signs of cirrhosis agrees with several cross-sectional and a few prospective studies in hemophilia indicating that only 20% to 30% of all hemophiliacs with chronic HCV infection ultimately develop cirrhosis.5,18However, considering that in patients with transfusion-transmitted hepatitis C HCC is a disease that may take up to 30 years to develop,19 in hemophiliacs this tumor might be less frequent than expected because these patients had a shorter survival than the nonhemophilic population as a consequence of the HIV epidemic.20,21
We also found that a direct relationship exists between the HCC risk and the age at which hemophiliacs became infected with HCV. In line with this observation are two recent studies in multitransfused patients showing a direct correlation between the age when patients become infected and the risk of developing a preneoplastic condition such as cirrhosis.22 23 Why hemophiliacs infected late in life are at a higher risk of HCC is unknown. An increased exposure to environmental factors responsible for cirrhosis or liver cancer or an increased vulnerability of the older liver to genotoxic agents are possible explanations.
The serum AFP level at enrollment was another important predictor of HCC risk in our patients that was identified by multiple regression and conditional risk ratio analysis. When the predictive power of serum AFP was assessed in a prospective fashion, it was found to be higher in population-based versus clinic-based studies, as a consequence of the many false-positive results in patients with cirrhosis.1 24In our setting, the predictive power of AFP was high, probably because of the low prevalence of patients with cirrhosis (10.3%).
The pattern of HCV genotypes among hemophiliacs with HCC paralleled the genotype distribution among Italian hemophiliacs, with a predominance of genotypes 1a and 3a, as reported elsewhere.25 Thus, genotype 1b, which in nonhemophilic patients with HCV-related cirrhosis was shown to be an important risk factor for HCC,26 in this study was not the predominant strain among HCV-infected hemophiliacs who developed HCC.
The main clinical goal of this study was to assess whether an annual screening with abdominal ultrasound examination and serum AFP measurement could help to reveal the tumor at an early, potentially treatable stage. Unfortunately, all six patients with HCC had multicentric disease at diagnosis, which was not amenable to surgical or radiologic treatment. The absolute rate of multicentric tumors in our patients is far higher than the prevalence (8% to 35%) reported in nonhemophilic cirrhotics.1,2,8,17 This might reflect differences in the natural history of HCC between hemophiliacs and nonhemophilic patients. In hemophiliacs, HCC may originate as multiple distinct clones of tumor disease as a consequence of multiple HCV infections acquired by multiple infusions of large-pool concentrates. However, since multiple infusions with allogeneic proteins may also impair immunity in these patients,11 12 HCC could initially develop as a monoclonal cancer and spread early within the liver to cause multinodal disease.
Whatever the biologic cause of multicentric HCC in hemophiliacs, it detracts from the potential utility of a screening program based on annual abdominal ultrasound examination and AFP assay, because multicentric tumors rarely respond to curative treatments. More aggressive schedules of screening might improve the early detection of HCC,24 27 and thus we are now conducting a prospective study of multitransfused hemophiliacs with chronic hepatitis C based on 6-month screening intervals.
ACKNOWLEDGMENT
Other members of the Study Group are as follows: F. Baudo, Talamona Division of Hematology, Niguarda Hospital, Milano; G. Castaman, Division of Hematology, Ospedale Civile, Vicenza; P.G. Mori, Division of Pediatrics, Gaslini Hospital, Genoa; A.R. Tagliaferri, 5th Division of Medicine, Hemostasis Disease Center, Policlinic Hospital, Parma; G. Muleo and R. Santoro, Hemophilia Center, Hematology Division, Ospedale Civile Pugliese, Catanzaro; and M. Schiavoni, Hemophilia and Thrombosis Center, Policlinic Hospital, Bari.
Supported by the Fondazione Italiana Ricerca Cancro.
Address reprint requests to M. Colombo, MD, Institute of Internal Medicine, University of Milan, Via Pace 9, 20122 Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.