Abstract
The gp55 envelope proteins of the spleen focus-forming virus initiate erythroleukemia in adult mice. Because the gp55 from the polycythemic strain (gp55-P), but not from the anemic strain (gp55-A), activates the erythropoietin receptor (EpoR) for proliferation of hematopoietic cell lines, the mechanism by which gp55-A initiates erythroleukemia has remained a mystery. We show here that gp55-A activates the EpoR in fetal liver cells. In contrast to previous studies using bone marrow cells from phenylhydrazine-treated, anemic mice, we find that both gp55-A and gp55-P induce erythroid differentiation from colony-forming unit-erythroid (CFU-E) progenitors in fetal liver cells. The effects on CFU-Es of both gp55-A and -P are mediated by the EpoR, because no colonies are seen upon expression of either gp55 in EpoR−/− fetal liver cells. However, only gp55-P induces erythroid bursts from burst-forming unit-erythroid progenitors and only gp55-P induces Epo independence in Epo-dependent cell lines. Using chimeric gp55 P/A proteins, we extend earlier work showing that the transmembrane sequence determines the capacity of gp55 proteins to differentially activate EpoR signaling. We discuss the possibilities for different signaling capacities of gp55-A and -P in fetal liver and bone marrow-derived erythroid progenitor cells.
THE PROLIFERATION, survival, and differentiation of erythroid progenitors into mature red blood cells (RBCs) absolutely requires erythropoietin (Epo) and the corresponding Epo receptor (EpoR).1,2 Epo−/− and EpoR−/− knock out embryos die around embryonic day 13 due to failure of fetal liver hematopoiesis. They contain normal numbers of both burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) progenitors, but the transition from CFU-E to mature RBCs is abolished. Thus, the EpoR is not required for generation of committed erythroid BFU-E and CFU-E progenitors, but is essential for terminal proliferation and differentiation of CFU-Es.1
The EpoR is a member of the type I cytokine receptor superfamily3,4 and is activated by ligand induced homo-dimerization.2,5-9 The EpoR activates a number of intracellular signal transduction pathways that are also activated by other cytokines and growth factor receptors, including JAK2, STAT5, PI-3′ kinase, and the protein tyrosine phosphatases SHP-1 and SHP-2.2,10 Exactly which signals or combination of signals are required for proliferation, survival, and differentiation of the various erythroid progenitors is not clear; however, these signals are likely to be shared by several cytokine receptors.11
The spleen focus-forming virus (SFFV) induces erythroleukemia in adult mice12 and the envelope glycoprotein of SFFV, gp55, is the only component required for oncogenicity.13,14 Two different strains of SFFV have been identified: the polycythemic (P) and the anemic (A).15 Although both induce erythroleukemia, SFFV-P induces polycythemia, a condition reminiscent of humanPolycythemia vera, and characterized by a very high number of mature RBCs. Early stages of disease are characterized by a dramatic increase in the number of erythroid BFU-E and CFU-E progenitors, which retain the capacity to terminally differentiate to mature RBCs and are not transplantable.16-18 Malignant clones emerge at 4 to 6 weeks after infection as a result of further genetic events. Specific to SFFV-induced leukemia is the activation of expression of the Spi-1 gene due to proviral insertional mutagenesis.19
In contrast, SFFV-A induces erythroleukemia with anemia. Because anemia is due to hemodilution and not to absolute loss of RBCs, the anemia strain may be called nonpolycythemic.20 gp55 sequences entirely account for the oncogenicity of both SFFV-A and -P, because they are oncogenic in the absence of other viral proteins.21 The phenotype of leukemia (P or A) is dependent on sequences at the 3′ end of the gp55 gene, which encodes the gp55 membrane spanning domain.22 The mechanism by which SFFV-A initiates erythroleukemia has remained a mystery,18because there is no evidence for a functional interaction between gp55-A and the EpoR, whereas several studies indicate that gp55-P likely activates the EpoR. For example, expression of gp55-P but not of gp55-A in interleukin-3 (IL-3)-dependent Ba/F3 cells results in growth-factor independence provided that the cells are expressing the EpoR.23 Interestingly, both gp55-P and gp55-A coimmunoprecipitate with the EpoR in these cells.24Similarly, gp55-P but not gp55-A renders Epo-dependent HCD 57 cells growth factor-independent.25 Bone marrow and spleen erythroid precursors isolated from mice infected with SFFV-A require Epo for in vitro CFU-E differentiation, whereas those infected by SFFV-P are Epo independent.26 Also, bone marrow-derived erythroid cells infected in vitro with SFFV-P can form erythroid bursts in the absence of Epo, whereas bone marrow-derived erythroid precursors infected with SFFV-A need Epo for maximal proliferation and for differentiation.27 We use wild-type (EpoR+/+) as well as EpoR−/− mouse embryos here to show that, in primary fetal liver cells, gp55-A indeed activates the EpoR and induces erythroid differentiation. Both gp55-A and gp55-P induce erythroid differentiation from CFU-E progenitors, and no colonies are formed upon expression of either gp55 in EpoR−/− fetal liver cells. However, only gp55-P induces erythroid bursts from BFU-E progenitors and only gp55-P induces Epo independence in Epo-dependent cell lines. Using chimeric gp55 P/A proteins, we extend earlier work showing that the transmembrane sequence determines the capacity of gp55 proteins to differentially activate EpoR signaling. Thus, within the fetal liver-derived CFU-E, signals delivered by the gp55-A through the EpoR are sufficient to support the terminal stages of erythroid proliferation and differentiation.
MATERIALS AND METHODS
Plasmids.
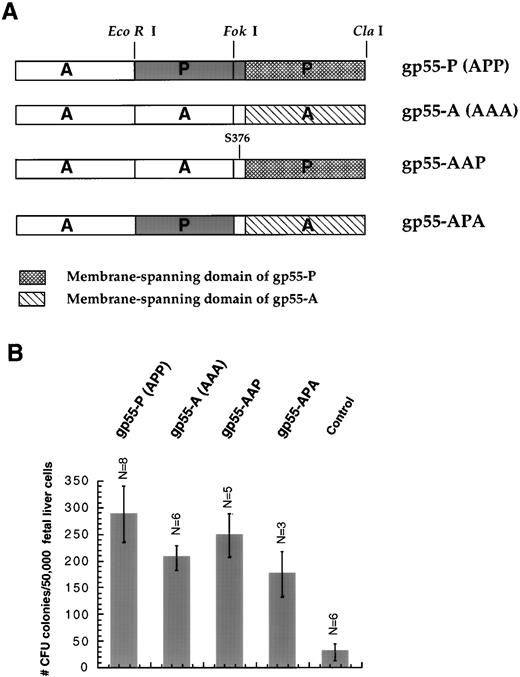
cDNAs encoding SFFV gp55 proteins, all cloned into SFFV cDNA, were kindly provided by Dr Sandra K. Ruscetti (National Cancer Institute, Frederick, MD). Restriction sites for EcoRI and Fok I divide the gp55 sequence into three fragments,22 labeled either A or P (Fig 1A). Thus, we denoted SFFVAP-L, which exerts effects identical to SFFV-P,14 as gp55-APP or, abbreviated, gp55-P. The construct denoted gp55-AAP contains only one residue (Ser 376) in the extracellular domain unique to gp55-P and the entire membrane-spanning domain of gp55-P (Fig 1A). Using primers encoding Bgl II andSal I restriction sites, gp55 sequences were polymerase chain reaction (PCR)-amplified and cloned into SFFV or into the BamHI and Sal I sites of pBABE and pMX retroviral vectors.28 29
(A) Diagram of the gp55 constructs. The EcoRI andFok I restriction sites were used to divide the coding sequence into three segments as described by Chung et al.22 P, sequences derived from gp55-P; A, sequences derived from gp55-A. gp55-APP was abbreviated as gp55-P because it is coded by SFFVAP-L, which induces polycythemic effects indistinguishable from those of SFFV-P.14 Construct gp55-AAP contains the entirety of gp55-A except for the membrane-spanning domain and one unique residue (Ser 376) in the exoplasmic domain derived from gp55-P. (B) Induction of erythroid colony formation from day-12.5 fetal liver CFU-E progenitors. Fetal liver cells were infected with retroviruses encoding gp55-P, gp55-A, gp55-AAP, or gp55-APA or with virus encoding β-galactosidase (control). Formation of CFU-E colonies was scored in the absence of Epo after 72 hours by staining with benzidine. Data represent the mean of the indicated number of assays (N) ± 1 standard deviation. Treatment with 1 U/mL Epo induced 957 ± 99 CFU-E colonies/50,000 fetal liver cells (N = 6).
(A) Diagram of the gp55 constructs. The EcoRI andFok I restriction sites were used to divide the coding sequence into three segments as described by Chung et al.22 P, sequences derived from gp55-P; A, sequences derived from gp55-A. gp55-APP was abbreviated as gp55-P because it is coded by SFFVAP-L, which induces polycythemic effects indistinguishable from those of SFFV-P.14 Construct gp55-AAP contains the entirety of gp55-A except for the membrane-spanning domain and one unique residue (Ser 376) in the exoplasmic domain derived from gp55-P. (B) Induction of erythroid colony formation from day-12.5 fetal liver CFU-E progenitors. Fetal liver cells were infected with retroviruses encoding gp55-P, gp55-A, gp55-AAP, or gp55-APA or with virus encoding β-galactosidase (control). Formation of CFU-E colonies was scored in the absence of Epo after 72 hours by staining with benzidine. Data represent the mean of the indicated number of assays (N) ± 1 standard deviation. Treatment with 1 U/mL Epo induced 957 ± 99 CFU-E colonies/50,000 fetal liver cells (N = 6).
Generation of retroviral supernatants.
To generate high-titer replication-free retroviral supernatants, we used transient transfection of the BOSC23 packaging cell line.30 BOSC cells were transfected using the calcium phosphate method with 5 μg of plasmid DNA encoding various gp55 constructs. As controls for transfection and infection, empty retroviral vectors or retroviruses encoding β-galactosidase, green fluorescence protein (GFP), or the cell-surface CD2 protein were transfected in parallel. Viral supernatants were collected 48 to 72 hours posttransfection and stored at −70°C. Viral titers were measured by infecting NIH 3T3 cells or Ba/F3 cells with serial dilutions of viral supernatant in the presence of 4 μg/mL polybrene and assaying by fluorescence-activated cell sorting (FACS) for cells exhibiting GFP fluorescence. Titers of 1 × 106 to 4 × 106 CFU/mL were routinely obtained. After collection of viral supernatants, expression of various gp55 proteins in the BOSC cells was assayed by Western blotting using the anti-gp55 7C10 monoclonal antibody (a gift of Dr Sandra Ruscetti) or with goat polyclonal anti-Rauscher gp70 envelope protein.
Erythroid colony formation in fetal liver cells.
Day-12.5 to -13.5 mouse fetal livers, which contain a majority of erythroid progenitors, were harvested and single-cell suspensions were prepared as described.1,11 Cells were infected with various retroviruses in the presence of 4 μg/mL polybrene for 4 hours.1,11 For CFU-E assays, 105 cells were resuspended in semisolid 1% methylcellulose medium containing 20% plasma depleted serum (Animal Technologies Inc, Tyler, TX), antibiotics, and β-mercaptoethanol or in MethoCult 3230 medium containing 0.9% methylcellulose and 20% fetal bovine serum (Stem Cell Technologies, Vancouver, British Columbia, Canada) in the presence or absence of 1 to 3 U/mL Epo (a generous gift from Dr Joan Egrie, Amgen Corp, Thousand Oaks, CA). Erythroid colonies generated by CFU-E progenitors were scored 72 hours after seeding by staining with diaminobenzidine (Sigma, St Louis, MO). As previously described,11 an aliquot of fetal liver cells infected in parallel with retroviruses encoding GFP or CD2 was incubated in liquid culture in Iscove's modified Dulbecco's medium (IMDM) containing 20% fetal calf serum and 1 U/mL Epo and analyzed 36 hours after infection using FACS scanning (Becton Dickinson, Mountain View, CA) for CD2 expression (using phycoerythrin-conjugated antimurine CD2 antibodies) or GFP expression to have a control for the efficiency of infection by a particular set of retroviruses. Colonies induced by expression of gp55 or Epo were photographed and scanned, and the surface occupied by CFU-E colonies was quantitated using the NIH Image program.
For BFU-E assays, 105 fetal liver progenitors were centrifuged after infection and resuspended in semisolid 1% methylcellulose medium containing 20% plasma derived serum, 1% spleen conditioned medium, and 50 ng/mL Steel factor (SF; a generous gift from Dr Joan Egrie) in the presence or absence of 3 U/mL Epo. Hemoglobinized BFU-E colonies were scored 7 to 9 days after infection.31 32 The capacity of gp55 proteins to replace SF or spleen-conditioned medium (SCM) was tested by omitting either one in the semisolid medium and testing the effects of Epo or gp55 on BFU-E formation in the presence or absence of Epo.
Assay for growth factor independence.
IL-3–dependent Ba/F3 cells stably transfected with the murine EpoR5 were grown in RPMI supplemented with 10% fetal calf serum and either 5% WEHI supernatant (as a source of IL-3) or 1 U/mL Epo. HCD 57 cells were grown in IMDM supplemented with 20% fetal calf serum and Epo.25 Cells were infected in the presence of 4 μg/mL polybrene with retroviral supernatants generated in BOSC cells and cultured in medium containing 1 U/mL Epo or 5% WEHI supernatant (as a source of IL-3) for 48 hours after infection. They were then extensively washed in RPMI and plated in growth medium without Epo or IL-3 in 24 wells of a microtiter plate. Viable growing cells were usually identified 4 to 6 days after growth factor withdrawal and cell pools were amplified and tested for expression of gp55 proteins by Western blotting. A minimum of three pools was analyzed for each construct. For viruses encoding GFP or CD2, FACS analysis was performed 24 and 48 hours after infection to have a control for the efficiency of a particular set of retrovirus infections.
Retroviral bicistronic expression of gp55 and GFP proteins.
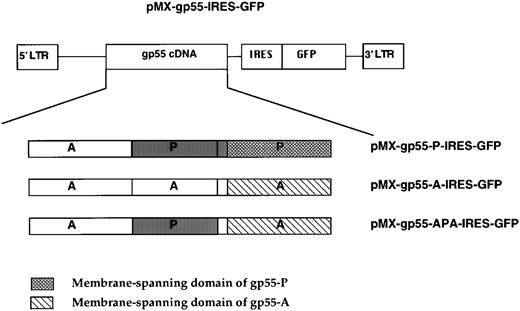
To achieve high levels of expression of transduced gp55 proteins that can be followed by FACS, we used GFP as a reporter gene. We generated a novel bicistronic retroviral vector, pMX-IRES-GFP, which will be described in detail elsewhere. Briefly, the encephalomyocarditis virus internal ribosome entry sequence (IRES) was inserted in front of the GFP gene in the pMX vector.29 Sequences for gp55-P (APP), gp55-A, or gp55-APA were cloned in front of the IRES sequence (see Fig5). The pMX-IRES-GFP constructs encoding gp55 proteins were transfected into BOSC23 cells and their expression was documented by FACS for GFP fluorescence and by Western blotting for gp55 expression. Retroviral supernatants generated from the transfected BOSC cells were used to infect Ba/F3 EpoR cells. The cells were cultured in WEHI (as a source of IL-3), and usually about 17% to 20% of the infected cells became fluorescent 24 hours after infection. The 1% most GFP fluorescent cells were sorted by FACS and then cultured in WEHI. As described in the text, these sorted cells were then assayed for their content of Epo- and IL-3–independent cells by culture in the absence of any growth factors. Epo-independent clones were analyzed by FACS and shown to express high levels of GFP. This technique allowed the isolation of cells stably expressing high levels of various gp55 proteins.
Bicistronic viruses encoding gp55 proteins and GFP.The pMX retroviral vector was modified to include the encephalomyocarditis virus IRES and, downstream, the GFP coding sequence. The cDNAs coding for gp55-P, gp55-APA, and gp55-A were inserted upstream of the IRES generating pMX-gp55-IRES-GFP vectors.
Bicistronic viruses encoding gp55 proteins and GFP.The pMX retroviral vector was modified to include the encephalomyocarditis virus IRES and, downstream, the GFP coding sequence. The cDNAs coding for gp55-P, gp55-APA, and gp55-A were inserted upstream of the IRES generating pMX-gp55-IRES-GFP vectors.
RESULTS
Activation of erythroid colony formation from CFU-Es by gp55 proteins.
Results obtained using Epo−/− and EpoR−/− mice showed that Epo is absolutely required for terminal proliferation and differentiation of fetal liver CFU-Es to mature erythrocytes.1 This led us to investigate whether expression of gp55 proteins can replace Epo and activate the EpoR to promote terminal proliferation and differentiation of CFU-E progenitors. We prepared retroviruses encoding gp55-P (or APP) and gp55-A (or AAA) as well as several gp55 chimeras, ie, gp55-APA and gp55-AAP (Fig 1A); gp55-AAP contains the entirety of gp55-A except for the membrane-spanning domain and one unique residue in the exoplasmic domain derived from gp55-P. The gp55 cDNAs were either cloned in SFFV22 or in the retroviral vector pBABE. We generated high-titer retroviral suspensions using the BOSC23 packaging cell system30 and used the virus to infect day-12.5 fetal liver cells. The fetal liver cells were assayed 2 to 3 days postinfection for erythroid colony formation from CFU-Es in the absence of Epo. This assay measures a combination of survival, proliferation, and differentiation in that a scored CFU-E colony has to contain at least 8 hemoglobinized cells.32
Figure 1B shows that expression of either gp55-P, gp55-A, gp55-AAP, or gp55-APA results in CFU-E differentiation in the absence of Epo. Two types of controls indicated that essentially all of the CFU-E progenitors infected by retroviruses expressing any of these gp55 proteins went on to form an erythroid colony. First, parallel cultures were infected with retroviruses encoding β-galactosidase or CD2 that were generated in parallel to those encoding gp55. An aliquot of cells from each infection was cultured for 36 hours in liquid medium in the presence of Epo and then analyzed by FACS scan for the fraction of infected cells. In repeated experiments, the ratio of the number of erythroid CFU-E colonies promoted by gp55 to that promoted (in uninfected cultures) by Epo was the same or higher than the estimated rate of retrovirus infection (15% to 25%); the latter was measured by the fraction of cells expressing transfected CD2 or β-galactosidase. Specifically, Epo supported the formation of 957 ± 99 CFU-E colonies per 50,000 nucleated fetal liver cells (N = 6; Fig 1); thus, the number of colonies (200 to 300 per 50,000 nucleated fetal liver cells; Fig 1B) induced by expression of gp55 proteins correlated very well with the frequency of infection.
As another means of determining the efficiency of retroviral infection, fetal liver cells infected with retroviruses encoding β-galactosidase were incubated in methylcellulose culture in the presence of Epo, and the resultant colonies were stained for β-galactosidase activity. The number of Epo-induced CFU-E colonies that expressed β-galactosidase (or CD2 in similar experiments) was the same (200 to 250 per 50,000 nucleated fetal liver cells) as the number of colonies induced by gp55 expression in the absence of Epo; again, the estimated frequency of infection was 15% to 25%. We conclude that most of the CFU-E progenitors infected by a retrovirus expressing either gp55-P or gp55-A undergo terminal erythroid differentiation in the absence of Epo.
Erythroid colonies induced by either gp55-P or gp55-A were significantly smaller than those induced by Epo. Most Epo-induced CFU-E colonies contained between 32 and 48 cells, whereas gp55-induced colonies contained between 12 and 16 cells. To better quantify these differences, we randomly photographed colonies induced by Epo or by gp55 and measured their surface using the NIH Image program. Colonies induced by gp55 had one half of the area of Epo-induced colonies. Specifically, the surface of Epo-induced colonies (N = 11) was 5,105 ± 662 arbitrary square pixels, whereas the surface of gp55-P promoted colonies was 2,649 ± 500 square pixels. In methylcellulose, the colonies are spherical; thus, it is possible that the twofold difference in surface area may represent an even more significant difference in volume.
Importantly, no difference in morphology, staining, or number of cells per colony was detected between those promoted by gp55-P and by gp55-A. Thus, gp55-P and gp55-A deliver an equivalent similar signal to fetal liver CFU-E progenitors that is sufficient to promote their terminal proliferation and differentiation into erythroid colonies.
Activation of erythroid burst formation from BFU-Es by gp55 proteins.
The in vitro assay for BFU-E erythroid differentiation measures the progression to CFU-Es and then to RBCs, a process that takes 7 to 9 days.32 In culture, differentiation of BFU-Es to CFU-Es requires Epo as well as IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF; both contained in SCM), or SF (KIT ligand). Thus, we infected day-12.5 to -13.5 fetal liver progenitors with retroviral suspensions encoding various gp55 proteins and then assayed for BFU-E erythroid differentiation by plating the cells in 1% methylcellulose in the presence of plasma-derived serum, SCM, and SF. Typical BFU-E colonies were scored 7 to 9 days after infection and stained with benzidine; alternatively, they were analyzed by Wright-Giemsa stain after cytospinning.
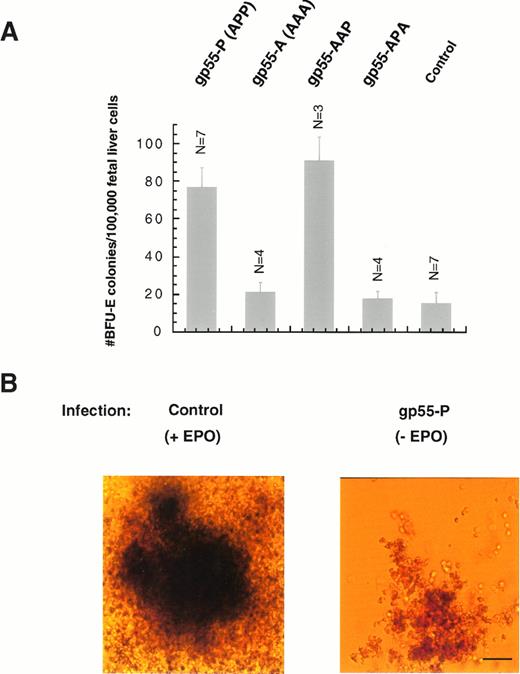
Figure 2A shows that expression of gp55-P was able stimulate BFU-E colony formation in the absence of Epo. As judged by the number of Epo-induced BFU-E colonies that expressed β-galactosidase, the estimated frequency of infection was 15% to 25%. The number of colonies induced by gp55-P infection and in the absence of Epo (∼80 per 100,000 nucleated fetal liver cells) was about one-third of that induced in control cultures treated with 3 U/mL Epo (230 colonies per 100,000 fetal liver cells). Thus, virtually every BFU-E infected by a retrovirus expressing gp55-P was able to form an erythroid burst. Figure 2B shows that the BFU-E colonies induced by gp55-P were significantly smaller than those induced by Epo; however, cytospin analysis and benzidine staining did not point to any differences other than in the numbers of cells, a result analogous to the situation with gp55-induced CFU-E colonies.
Induction of erythroid burst formation from day-12.5 fetal liver BFU-E progenitors. (A) Fetal liver cells were infected with retroviruses encoding gp55-P, gp55-A, gp55-AAP, or gp55-APA or with virus encoding β-galactosidase (control). Formation of BFU-E colonies was scored 7 to 9 days after infection in complete methylcellulose medium containing plasma-derived serum, SCM, and SF (see the Materials and Methods). Data represent the mean of the indicated number of assays ± 1 standard deviation. Treatment with 3 U/mL Epo resulted in the formation of 230.5 ± 16.1 BFU-E colonies per 100,000 fetal liver cells (N = 4). (B) BFU-E colonies induced either by 3 U/mL Epo or after infection with SFFV-gp55-P and culture in the absence of Epo. Scale bar = 50 μm.
Induction of erythroid burst formation from day-12.5 fetal liver BFU-E progenitors. (A) Fetal liver cells were infected with retroviruses encoding gp55-P, gp55-A, gp55-AAP, or gp55-APA or with virus encoding β-galactosidase (control). Formation of BFU-E colonies was scored 7 to 9 days after infection in complete methylcellulose medium containing plasma-derived serum, SCM, and SF (see the Materials and Methods). Data represent the mean of the indicated number of assays ± 1 standard deviation. Treatment with 3 U/mL Epo resulted in the formation of 230.5 ± 16.1 BFU-E colonies per 100,000 fetal liver cells (N = 4). (B) BFU-E colonies induced either by 3 U/mL Epo or after infection with SFFV-gp55-P and culture in the absence of Epo. Scale bar = 50 μm.
Infection with viruses encoding gp55-A did not result in erythroid burst formation (Fig 2A and Table 1). Thus, the BFU-E assay clearly differentiates between the polycythemic (P) and anemic (A) phenotype. Because gp55-A and gp55-APA did not induce BFU-E differentiation and because gp55-P and AAP did induce normal BFU-E differentiation (Fig 2A and Table 1), sequences located within the membrane-spanning domain are responsible for the differences between gp55-P and gp55-A. This correlates very well with the capacity of several chimeric gp55 proteins to induce polycythemia in adult mice.25 Furthermore, because the gp55-AAP chimera contains only one unique residue from the exoplasmic domain of gp55-P (at position 376, Fig 1A) and the entire membrane-spanning domain of gp55-P, the notion that the transmembrane domain is crucial for the observed effects between gp55-P and gp55-A is strengthened.
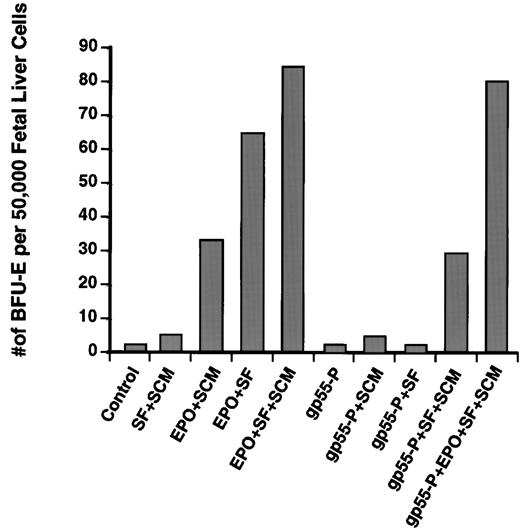
For Epo to stimulate maximum BFU-E colony formation from fetal liver progenitors, the medium must contain SF and SCM, which provides a mixture of IL-3 and GM-CSF. Whereas Epo alone cannot support BFU-E colony formation (not shown), a combination of Epo and SF (lacking SCM) induced formation of 76% of the number of BFU-E colonies induced by complete medium (Epo + SF + SCM; Fig 3), and a combination of Epo and SCM induced 40% of the number of colonies induced by complete medium.Thus, Epo can induce a significant number of BFU-E colonies in the presence of only SF or only SCM. In contrast, gp55-P requires the presence of both SF and SCM in the plating medium to promote BFU-E differentiation. (Fig 3). The smaller size of BFU-E colonies induced by gp55-P and the absolute requirement for both SF and SCM for gp55-P to stimulate BFU-E differentiation suggest that gp55-P can only partially activate the EpoR in BFU-Es.
Induction of erythroid burst formation from day-12.5 fetal liver BFU-Es by gp55-P require the presence of both SF and SCM. Fetal liver cells were infected with retroviruses encoding gp55-P or, as control, β-galactosidase and then plated in methylcellulose containing the indicated growth factors. Data represent the average of two typical experiments, each performed in duplicate; the variation was less than 20%. Cells plated solely in Epo without SF and SCM did not produce any BFU-E colonies.
Induction of erythroid burst formation from day-12.5 fetal liver BFU-Es by gp55-P require the presence of both SF and SCM. Fetal liver cells were infected with retroviruses encoding gp55-P or, as control, β-galactosidase and then plated in methylcellulose containing the indicated growth factors. Data represent the average of two typical experiments, each performed in duplicate; the variation was less than 20%. Cells plated solely in Epo without SF and SCM did not produce any BFU-E colonies.
Expression of the EpoR and the effects of gp55 proteins on erythroid differentiation.
Because expression of either gp55-P or gp55-A can replace Epo for the activation of CFU-E differentiation in fetal liver cells, we investigated whether expression of the EpoR was required for this function. To this end, we infected day-12.5 fetal liver cells from homozygous EpoR−/− embryos with viruses encoding gp55 proteins and then assayed for erythroid colony formation from CFU-E progenitors as previously described.1,33 In this system, retroviral-mediated expression of the EpoR results in Epo- and SF-dependent formation of erythroid colonies from CFU-E and BFU-E progenitors at 72 hours and 7 to 9 days postinfection, respectively.1,33 Figure 4A shows that none of the gp55-expressing viruses (cloned in SFFV or pBABE) was able to induce erythroid colony formation from EpoR−/− CFU-E progenitors. In these experiments, SF was added to all samples as a precaution, because an interaction between the KIT and Epo receptors is essential for terminal differentiation of EpoR−/− CFU-E progenitors expressing recombinant EpoR.33 In one control experiment, infection of the EpoR−/− progenitors with virus encoding the EpoR resulted in Epo-dependent formation of a large number of erythroid colonies in the presence of SF (Fig 4A, right column). As another control, the same gp55-encoding retroviral suspensions were able to induce CFU-E differentiation from wild-type (EpoR+/+) fetal liver cells (Fig 4B). Similar results (not shown) were obtained when differentiation from BFU-E progenitors was assayed, showing that expression of gp55-P results in erythroid colony formation only in the presence of the EpoR.
Induction of erythroid colony formation from fetal liver CFU-E progenitors requires the expression of the EpoR. Day-12.5 fetal liver cells dissected from EpoR−/− embryos (A) or from wild-type embryos (B) were infected with viruses encoding β-galactosidase (control), gp55-P, gp55-A, and murine EpoR. The retroviral vector used is indicated (SFFV or pBABE). Cells were assayed in CFU-E assays in the absence or presence of EPO or (in the right-most panel of [A]) in the presence of Epo and SF. Data represent the average of two experiments, each performed in duplicate; the variation was less than 20%.
Induction of erythroid colony formation from fetal liver CFU-E progenitors requires the expression of the EpoR. Day-12.5 fetal liver cells dissected from EpoR−/− embryos (A) or from wild-type embryos (B) were infected with viruses encoding β-galactosidase (control), gp55-P, gp55-A, and murine EpoR. The retroviral vector used is indicated (SFFV or pBABE). Cells were assayed in CFU-E assays in the absence or presence of EPO or (in the right-most panel of [A]) in the presence of Epo and SF. Data represent the average of two experiments, each performed in duplicate; the variation was less than 20%.
gp55-P and not gp55-A induce Epo-independent growth of Epo-dependent cells: bicistronic expression of gp55 and GFP.
Expression of gp55-P but not gp55-A induces growth-factor independence of certain Epo-dependent hematopoietic cell lines.23,25,34We tested viruses encoding gp55-P, gp55-A, and gp55-APA for their ability to induce Epo-independence in Ba/F3-EpoR or HCD 57 cells. Whereas gp55-P, and gp55-AAP induced Epo-independence, gp55-PPA, gp55-A, and gp55-APA did not (Table 1). Thus, sequences located primarily within the membrane-spanning domain of gp55 are crucial for induction of Epo-independence, confirming previous work,25as well as for fetal liver BFU-E differentiation.
Because only gp55-P and chimeras of gp55 that contain the membrane-spanning domain of gp55-P can render cell lines Epo independent, we were not able to analyze signaling events induced by gp55-A or gp55-APA. Furthermore, in factor-independent cells expressing gp55-P, one is studying events that may be a consequence of the selection for Epo independence and not those due only to expression of gp55-P. Indeed, whereas virtually all BFU-E and CFU-E progenitors infected with a retrovirus encoding gp55-P were able to form an erythroid colony or burst in the absence of Epo (Figs 1 and 2), our preliminary experiments indicated that only a minority of EpoR-expressing Ba/F3 cells infected with a retrovirus encoding gp55-P was able to proliferate in the absence of Epo.
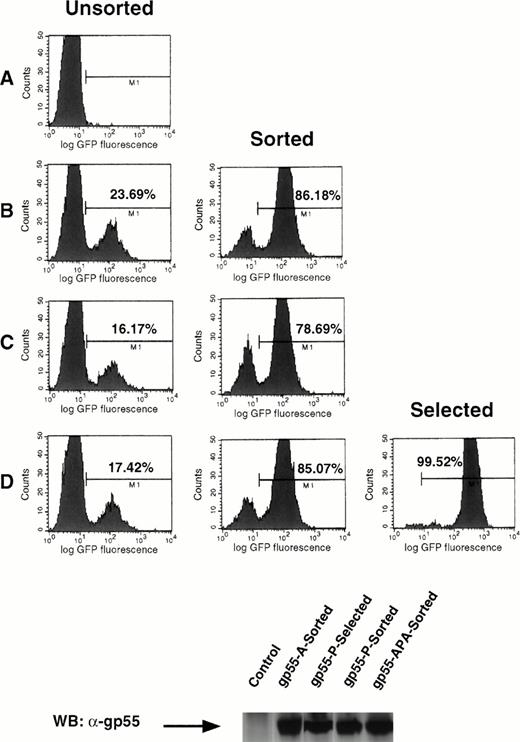
To understand better the relationship between the efficiency of infection and the induction of Epo independence in cell lines, we used a bicistronic retroviral vector encoding a gp55 protein and GFP, separated by an IRES (Fig 5 and the Materials and Methods). In this system, the translation of the two proteins is tightly linked, in that expression of GFP (initiated by the IRES) is proportional over a 100-fold range to the level of expression of the protein coded by the cDNA directly downstream of the left LTR.35 We generated retroviruses encoding gp55-P, gp55-A, and gp55-APA by packaging pMX-gp55-IRES-GFP constructs in BOSC 23 cells and used these to infect Ba/F3 EpoR cells (Fig 6). Cells were scanned by FACS for GFP fluorescence and sorted for high levels of GFP fluorescence. Pools of sorted cells expressing the same amounts of gp55-A-GFP, gp55-APA-GFP, and gp55-P-GFP (Fig 6B, C, and D, respectively) were assayed for the capacity to grow in the absence of Epo. Only gp55-P-GFP cells were able to grow after Epo withdrawal. As an important control, the levels of gp55 protein were similar in cells sorted for GFP expression and expressing gp55-P, gp55-A, or gp55-PPA; these levels were comparable to that in gp55-P–infected cells and selected for growth in the absence of Epo (Fig 6, lower panel).
Bicistronic expression of gp55 proteins and GFP. Ba/F3 EpoR cells were infected with pMX-gp55-IRES-GFP retroviruses encoding gp55-A (B), gp55-APA (C), and gp55-P (D) cloned in the pMX-IRES-GFP retroviral vector or with a similar pMX retrovirus without an insert (A). The FACS analysis shows the percentage of cells that express different levels of GFP at 48 hours after infection. Cells with high GFP fluorescence (bars in B, C, and D) were sorted and then cultured in the presence of Epo. Greater than 78% of these cells retained high GFP expression, as indicated by the panels in the middle column depicting the sorted cells. These sorted cells were also assayed for Epo independence. Only cells infected with pMX-gp55-P-IRES-GFP virus could grow in the absence of Epo; FACS analysis of these is shown in (D), Selected. The level of expression of gp55 proteins in the respective cell lines was shown in the lower panel by a Western blot of whole cell lysates with the anti-gp55 antibody.
Bicistronic expression of gp55 proteins and GFP. Ba/F3 EpoR cells were infected with pMX-gp55-IRES-GFP retroviruses encoding gp55-A (B), gp55-APA (C), and gp55-P (D) cloned in the pMX-IRES-GFP retroviral vector or with a similar pMX retrovirus without an insert (A). The FACS analysis shows the percentage of cells that express different levels of GFP at 48 hours after infection. Cells with high GFP fluorescence (bars in B, C, and D) were sorted and then cultured in the presence of Epo. Greater than 78% of these cells retained high GFP expression, as indicated by the panels in the middle column depicting the sorted cells. These sorted cells were also assayed for Epo independence. Only cells infected with pMX-gp55-P-IRES-GFP virus could grow in the absence of Epo; FACS analysis of these is shown in (D), Selected. The level of expression of gp55 proteins in the respective cell lines was shown in the lower panel by a Western blot of whole cell lysates with the anti-gp55 antibody.
This system allowed us to determine that the fraction of gp55-P–expressing cells that eventually becomes Epo independent is only 1 in 1,000 to 1 in 5,000. This explains the lag phase of 4 to 6 days required for generation of Epo-independent clones of Ba/F3 cells after infection with gp55-P coding viruses.23 36 In contrast, virtually all fetal liver progenitors infected with a gp55-P–expressing virus formed erythroid colonies and bursts (Figs 1and 2).
DISCUSSION
Our most important result is that both types of SFFV envelope proteins, polycythemic (gp55-P) and anemic (gp55-A), promote erythroid colony formation from fetal liver CFU-Es in the absence of Epo. Colony formation requires expression of the EpoR, a result that implies that gp55-A indeed can activate the EpoR and that any quantitative or qualitative differences in signaling between gp55-P and gp55-A are irrelevant at this stage of erythroid differentiation. We also showed that gp55-P but not gp55-A can promote erythroid colony formation from BFU-E progenitors and that only gp55-P can induce growth factor independence in Epo-dependent cell lines. To insure that the inability to obtain factor-independent cells expressing gp55-A was not due to absence of gp55-A expression, we used a bicistronic retrovirus vector expressing GFP under control of an IRES to generate populations of Ba/F3 EpoR cells stably expressing gp55-P and gp55-A at high levels; again, only gp55-P–expressing cells became Epo independent. Consistent with these observations, recent studies showed that, in transfected Ba/F3 cells expressing the EpoR, the levels of cell-surface gp55-P and gp55-A proteins are similar but that the interaction of the two gp55 proteins with the Epo receptor is different; only gp55-P could be cross-linked to radioiodinated Epo.37 Finally, by using chimeric gp55 A and P proteins, we showed that only the membrane-spanning segment of gp55-P is essential for promoting erythroid colony formation from BFU-E progenitors as well as for induction of growth factor independence in Epo-dependent cell lines.
To study the functional interaction between gp55 proteins and the EpoR, we used fetal liver erythropoiesis. In this system, we can follow erythroid differentiation from BFU-E to CFU-E and finally to RBCs and we can assess the role of the EpoR by parallel experiments in EpoR−/− embryos. Earlier studies were performed with bone marrow progenitors taken from adult mice that had been rendered anemic by phenylhydrazine27,38 or with bone marrow and spleen erythroid precursors derived from mice infected with SFFV-A or SFFV-P.26 These studies showed that gp55-A stimulated the proliferation but not the terminal differentiation of BFU-Es, whereas gp55-P was able to replace Epo for both proliferation and differentiation.27,38 No effect on CFU-E differentiation was reported when gp55-expressing retroviruses were used to infect, in vitro, bone marrow cells.27,38 However, bone marrow CFU-Es isolated from SFFV-P–infected animals were shown to form erythroid colonies in the absence of Epo, whereas precursors isolated from SFFV-A–infected animals still required Epo for erythroid differentiation.26 We can suggest several explanations for the discrepancies between these studies and our present results.
First, although Epo induces CFU-E differentiation in bone marrow progenitors, the effects of in vitro infection with SFFV-P seem to be uniquely on the BFU-Es, which form bursts earlier (by day 5) than do the Epo-induced bursts (day 8).38 Second, the experiments on bone marrow progenitors were performed using replication competent Friend virus; we used replication-incompetent viruses. Third, there may be important overlooked differences between EpoR signaling in fetal liver versus adult bone marrow progenitor cells. Indeed, the transcription factor PU.1 is required for erythropoiesis in adult bone marrow progenitors but not in fetal liver cells; ES cells homozygous for a PU.1 deletion (PU.1−/− knock out cells), when injected into PU.1+/+ embryos, contribute to fetal liver-derived erythrocytes but not to bone marrow-derived erythrocytes, and PU.1−/− fetal liver cells can reconstitute the erythroid compartment of lethally irradiated adult mice.39 Furthermore, fetal liver hematopoietic stem cells (HSC) are functionally and phenotypically different from bone marrow HSC.40 Fetal liver HSC include a much higher frequency of cycling cells and have higher reconstitution ability in lethally irradiated mice when compared with bone marrow HSC.40Together with the data from previous studies in bone marrow,26,27 38 our data show that differences between fetal liver and bone marrow progenitors can also be noted at the later, committed CFU-E stage.
Our studies, using high-titer replication-defective recombinant retroviruses and fetal liver progenitor cells derived from normal mouse embryos, indicate that gp55-P and gp55-A are equally able to activate the EpoR for proliferation and terminal differentiation of CFU-E progenitors. Thus, the suggestion27 that gp55-A cannot activate a differentiation-specific pathway is unlikely, at least in the context of fetal liver CFU-Es. These results point to interesting differences between fetal liver and bone marrow progenitors that deserve further study.
The CFU-E and the BFU-E colonies induced by gp55 proteins were smaller than those induced by Epo, due to the presence of lower numbers of cells per colony. Epo-stimulated proerythroblasts both proliferate (ie, generate more proerythroblasts) and divide to progress toward terminal differentiation. The cell divisions required for progression towards reticulocytes are also called differentiation divisions, because proerythroblasts normally divide 4 to 5 times to generate progressively smaller erythroblast intermediates and eventually to generate reticulocytes.41 Our cytospin analysis showed the presence of the expected erythroblast intermediates in the gp55-A– and gp55-P–induced colonies. In contrast, Epo-induced colonies apparently contain erythroblast intermediates that also undergo proliferation before dividing to yield the next intermediate in the differentiation program. We hypothesize that such proliferation of CFU-Es is stimulated by Epo but not by gp55-P or gp55-A proteins. Our study does show that the effect of gp55 in primary cells is due to the EpoR because, in EpoR−/− knock out embryos, neither gp55-P nor gp55-A can induce CFU-E colonies, irrespective of the presence of SF in the plating medium.
Interestingly, expression in EpoR−/− fetal liver cells of a mutant EpoR containing only 1 of the 8 cytosolic tyrosines (F7Y401) resulted in the formation of a normal number of small CFU-E colonies33 similar to the ones induced by gp55-A and gp55-P. Similar to gp55-A, EpoR mutant F7Y401 cannot support Epo-dependent proliferation of Ba/F3 cells.33 We suggest that, in CFU-E cells, gp55 delivers an incomplete signal through the EpoR that activates some signal transduction pathways (hypothetically, one[s] activated through phosphorylated tyrosine 401) but not others, yet still triggers the differentiation divisions. Whether gp55 activates tyrosine phosphorylation of EpoR tyrosine 401 in CFU-E cells is under investigation in our laboratory. Erythroid differentiation requires a fine balance between the levels of GATA transcription factors, and this level may be differentially affected by Epo or gp55 activation of the EpoR. A low level of expression of the GATA2 transcription factor (or a low GATA2/GATA1 ratio) was associated with erythroid differentiation and small-sized bursts.42
A high level of expression of gp55-P is not sufficient to induce autonomous cell growth of Ba/F3 EpoR cells. Using a bicistronic retrovirus to coexpress gp55 proteins with GFP, we showed that only a tiny minority of cells expressing gp55-P became Epo independent. This finding is consistent with the several-day lag period required for detection of factor-independent growth after infecting EpoR-expressing cell lines with viruses expressing gp55-P. This bicistronic retrovirus system may be useful to study the initial stages of receptor activation by gp55 proteins, in the absence of selection for Epo-independent growth. For example, we recently showed that cells sorted for high levels of gp55-P expression indeed exhibit factor-independent activation of STAT5, whereas cells expressing equivalent high levels of gp55-A or gp55-PPA do not, suggesting that activation of STAT5 is a direct consequence of gp55-P expression (unpublished results). This system will enable further study of the initial events required for gp55-expressing cells to escape dependence on growth factors.
Finally, although both gp55-P and gp55-A activate the EpoR, the nature of these interactions is different. Because gp55-A activated the combination of cell proliferation and differentiation required for colony formation from CFU-E progenitors, we suggest that the differences between gp55-A and gp55-P are quantitative rather than qualitative. At the CFU-E stage, the proliferation and differentiation programs are coupled and can be initiated by much weaker signals from the EpoR than those required at the earlier late BFU-E stage. This is in agreement with the significantly lower concentrations of Epo required for erythroid differentiation in vitro of CFU-Es than from BFU-Es.43 EpoR-expressing cell lines appear to be quite insensitive to gp55-P, because only a minority of cells expressing gp55-P eventually became autonomous for cell growth. Thus, our new data provide novel insights into the mechanisms of EpoR signaling and also point to important differences in EpoR signaling between fetal liver and bone marrow-derived erythroid progenitors.
ACKNOWLEDGMENT
The authors thank Dr Sandra K. Ruscetti (Laboratory of Molecular Oncology Frederick Cancer Research and Development Center, National Cancer Institute, Frederick, MD) for gp55 cDNA constructs and for monoclonal antibody 7C10. We thank Dr Merav Socolovsky for advice on retroviral infection of fetal liver cells and for many suggestions.
Supported by Grant No. HL 32262 from National Institute of Health and by a grant from Amgen Corporation to H.F.L. S.N.C. held a fellowship from the Anna Fuller Fund and is now a fellow of the Medical Foundation/Charles A. King Trust. X.L. is supported by a postdoctoral fellowship from the National Institutes of Health. H.W. was supported by a postdoctoral fellowship from the Damon Runyan-Walter Winchell Cancer Research Fund.
Address reprint requests to Harvey F. Lodish, PhD, Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. Induction of erythroid colony formation from fetal liver CFU-E progenitors requires the expression of the EpoR. Day-12.5 fetal liver cells dissected from EpoR−/− embryos (A) or from wild-type embryos (B) were infected with viruses encoding β-galactosidase (control), gp55-P, gp55-A, and murine EpoR. The retroviral vector used is indicated (SFFV or pBABE). Cells were assayed in CFU-E assays in the absence or presence of EPO or (in the right-most panel of [A]) in the presence of Epo and SF. Data represent the average of two experiments, each performed in duplicate; the variation was less than 20%.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1163/4/m_blod4044104.jpeg?Expires=1768867823&Signature=HKnobt0yO-cjYM8l6CS3LVR-Jvv1zpDC62InpumBkeIgywEacANuSWn3PVxxkztEayhZ6XbmPoJ-ekFGTfCgpfFK9lYbc5wtukOtx7XSrp48XanNWmuXr2C3hvP5bCBjd8tV4Rl3x3wqHNCbGVBLsGu3z4Z9Pm5sdYFYKp3~bzVm-XwVR82vcYAjvV92VVruuUKOWcNHQj~eRbP8gF4Y7nI76PxX9BvtHnp56WB9OteJLXUIqhFhhVnnRfTNQekSbYaCz1Xx-~82LsBcMFCfPrXxo-bydHIxW-bkLPE7bgci35AW3e1~kw8YaDYl9xfMeT3WA1u5gHm4KvPi2EUqxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)