Abstract
Of 260 patients enrolled, 25 patients (9.6%) were associated with acquired von Willebrand syndrome (AvWS). We studied 25 patients with AvWS, retrospectively. AvWS was diagnosed by reduced levels of von Willebrand factor (vWF) (decrease of von Willebrand factor antigen [vWF:Ag] and von Willebrand ristocetin cofactor [vWF:RCoF]), a decrease of ristocetin-induced platelet agglutination (RIPA), sometimes decreased high-molecular-weight multimers, and prolonged bleeding time with neither prior nor family histories of bleeding problems and the evidence of normal vWF:RCoF in their families. The inhibitor of vWF was determined by mixing patient plasma with pooled normal plasma. Eight patients in this study had the inhibitors to vWF that were of the IgG class; the subclasses were IgG1 (7 cases) and IgG2 (1 case). Multimeric analysis of vWF showed selective loss of large multimers in most patients with AvWS similar to that of congenital type-2 von Willebrand disease (vWD). All inhibitors blocked ristocetin-mediated vWF binding to platelets. Five out of 6 IgGs evaluated here recognized the 39/34-kD fragment (residues 480/481-718) and Fragment III (residues 1-1365) that implied binding domain of glycoprotein Ib (GPIb), whereas 1 recognized Fragment I (residues 911-1365). A close relationship was found between the presence of the inhibitor and bleeding tendency. Of the 7 patients with inhibitors, 6 patients (86%) had a bleeding tendency, as well as 1 of the 15 patients without inhibitors (6%). The efficacy of treatment of underlying diseases and/or therapy with deamino D-arginine vasopressin (DDAVP) for the treatment of AvWS also depends on the presence of an inhibitor. Four of 8 patients with inhibitors (50%) had poor response to treatment of the underlying disease and/or therapy with DDAVP, as well as 1 of the 16 patients without inhibitors (6%). These results indicate that patients with AvWS developing inhibitors to vWF are likely to have bleeding problems and might be resistant to treatment of underlying diseases and/or therapy with DDAVP for bleeding to AvWS. We also showed evidence that intravenous immunoglobulin therapy (0.3 g/kg, 3 days) was effective to correct a hemostatic defect and manage severe bleeding in a patient with AvWS developing inhibitors. We might consider an additional treatment including expensive high-dose immunoglobulin therapy when uncontrollable bleeding is continued after the treatment of the underlying diseases and/or therapy with DDAVP.
VON WILLEBRAND FACTOR (vWF) is a multimeric plasma glycoprotein that is involved in adhesion and aggregation of platelets at the site of vascular injury.1Its key functions include binding to the platelet membrane receptors, glycoprotein (GP) Ib and IIb/IIIa.2 Acquired von Willebrand syndrome (AvWS) results from quantitative and/or qualitative defects in vWF mimicking the clinical and laboratory features of hereditary von Willebrand disease (vWD). The incidence of AvWS in various diseases has not been clearly known. A recent prospective study unexpectedly found AvWS in 8% of Wilms tumor patients.3Laboratory examination shows that bleeding time is normal or prolonged and ristocetin cofactor activity (vWF:RCoF) is characteristically low or nearly absent, whereas von Willebrand factor antigen (vWF:Ag) is low or normal. Furthermore, multimeric analysis of vWF most commonly shows a pattern similar to type-2 congenital vWD, with selective loss of high molecular weight multimers.4
AvWS is reported in association with monoclonal gammaglobulins, lymphoproliferative disorders such as lymphomas, myelomas, Wilms' tumors and autoimmune disorders, suggesting that deficiency of vWF has an immunological etiology. Two mechanisms have been proposed to explain the pathogenesis of the acquired deficiency of vWF in these cases: (1) the presence of circulating antibodies that inactivate functional domains of vWF or form a complex with vWF by binding to its functional sites, the bound vWF is rapidly cleared from the circulation5-8 (however, antibodies to vWF are shown in vitro in only a minority of the cases); and (2) selective absorption of large and intermediate multimers of vWF by malignant cells9(however, in the majority of patients the mechanism of the vWF deficiency has not been clearly delineated).
Treatment of AvWS depends on the underlying disease process and the mechanisms responsible for development of the syndrome. The treatment of underlying diseases is crucial for treatment of AvWS. However, it is not possible to predict which patients will have a good response to the treatment of underlying diseases and/or therapy with deamino D-arginine vasopressin (DDAVP). We hypothesized that the presence of the inhibitor predicts the response to the treatment of AvWS.
In this paper we described a series of 25 patients with AvWS to evaluate the laboratory features, possible pathogenetic mechanisms for AvWS, and therapeutic strategies for the treatment of AvWS. We found that the presence of inhibitors was a sensitive and specific predictor of both the response to treatment of the underlying diseases and/or therapy with DDAVP, and bleeding tendency.
PATIENTS AND METHODS
Patients.
From January 1985 to December 1996 at Yokohama City University School of Medicine, Yokohama, Japan, we have studied 260 patients with various disorders; 125 patients with chronic myeloproliferative disorders (chronic myelocytic leukemia [CML], 57 patients; polycythemia rubra vera [PV], 37 patients; essential thrombocythemia [ET], 31 patients), 51 patients with non-Hodgkin's lymphoma (NHL), 42 patients with multiple myeloma (MM), 28 patients with acute leukemia (acute myelocytic leukemia [AML], 15 patients; acute myelomonocytic leukemia [AMMoL], 7 patients; acute lymphocytic leukemia [ALL], 6 patients), and 14 patients with chronic lymphocytic leukemia (CLL) (stage II, 4 patients; stage III, 5 patients; stage IV, 5 patients). The diagnosis of CML was confirmed by the cytogenetic finding of the Ph1chromosome. All patients with CML were in chronic phase. PV was diagnosed by the criteria described elsewhere.10 The criteria of ET were based on abnormal proliferation of the megakaryocytes and platelet count more than 106/mm3 with splenomegaly.11 The diagnosis of lymphoma was confirmed by lymph node biopsy and lymphomas were classified as non-Hodgkin's lymphoma or Hodgkin's disease according to the Rye classification.12 The diagnosis of MM was established according to the criteria of the Southwest Oncology Group.13 Acute leukemia was classified according to the French-American-British classification.14 Subclasses of AML were based on morphological and cytochemical criteria. The diagnosis of CLL was based on clinical, morphological, and immunophenotypic criteria as described elsewhere.15
Study design.
The criteria for diagnosis of AvWS were reduced levels of vWF (decrease of vWF:Ag and vWF:RCoF), a decrease of ristocetin-induced platelet agglutination (RIPA), sometimes decreased high-molecular-weight multimers and prolonged bleeding time with no prior history of bleeding problems and a family history negative for a bleeding tendency, whereas that of inherited vWD (except for type 2B and platelet type) were those with family history.16 In patients with type-2B and platelet-type vWD, RIPA showed increased sensitivity. To exclude inherited vWD, blood samples from some family members were tested for vWF:Ag, vWF:RCoF, and RIPA. ABO blood type was also evaluated.
All patients were evaluated before treatment. Blood samples were obtained from all patients and tested for platelet count, vWF:Ag, vWF:RCoF, and RIPA. The tests were performed by laboratory staff without knowledge of the patients' clinical presentations. Inhibitors of vWF were also determined by a mixing study that was performed by incubation of patient plasma with pooled normal plasma.
Laboratory studies.
The bleeding time was measured by the modified method of Ivy17 using an automatic template device (Simplate; General Diagnostic, Morris Plains, NJ). The normal laboratory range for bleeding time is 4 to 8 minutes. vWF antigen (vWF:Ag) was evaluated by the Laurel technique of quantitative immunoelectrophoresis.18 The normal range for both factors in our laboratory is 50% to 150%. vWF:RCoF was assayed with formalin-fixed platelets as described elsewhere.19 The normal range is 60% to 150%. The multimeric composition of vWF was analyzed by sodium dodecyl sulfate (SDS) 0.9% agarose gel electrophoresis according to the method described elsewhere.20
Platelet aggregation was performed in siliconized cuvettes at 37°C and stirred at 1,000 rpm as described previously.21Ristocetin (1.2 mg/mL) was added to platelet-rich plasma. Aggregation was recorded as the increase in the light transmission through the mixture.
Assay of an inhibitor against FVIII:C was performed as described elsewhere.22 Inhibitors of vWF were sought by incubating 1 volume of patient plasma with 1 volume of pooled normal plasma for 1 hour at 37° as described elsewhere.5 23 Controls were determined by mixture of normal plasma (1 volume) with phosphate-buffered saline (PBS) (1 volume). Patient and normal plasmas were also incubated independently under the same conditions. The expected values were calculated from the values for patient plasma and normal plasma incubated and assayed separately.
The Ig class of the inhibitors was identified as previously described.5 23 In brief, serum from the patients was added in increasing concentration to the wells coated with vWF for 1 hour at 22°. After washing with PBS, 0.05% Tween 20, horseradish peroxidase (HRP)-conjugated goat Igs to either human IgG (Zymed Inc, South San Francisco, CA), IgM, or IgA (Bioscience, Copenhagen, Denmark) was added to each well. Then the peroxidase substrate (o-phenylenediamine; Zymed) was added and the reaction was blocked with 2 mol/L H2SO4.
Human vWF was produced from cryoprecipitate (a kind gift from Dr Zaverio M. Ruggeri; Scripps Research Institute, La Jolla, CA) and was also purchased from Bio Pur AG (Bubendorf, Switzerland). Human plasma fibronectin was purchased from Bachen Chemical Co, Ltd (Torrance, CA). A monomeric 39/34-kD fragment of human vWF (residues 480/481-718) was obtained by dispase digestion of purified vWF and purified by a heparin-Sepharose affinity chromatography as described elsewhere.24 This fragment is crucial for the binding to GPIb. Fragment I (residues 911-1365), Fragment II (residues 1366-2050), and Fragment III (residues 1-1365) were generated by digestion of vWF with Staphylococcus aureus V8 protease (SV8) and were characterized as described previously.25
To evaluate the effect of the inhibitor on the ristocetin-mediated vWF-GPIb interaction, 125I-vWF (2 μg/mL) was incubated with formalin-fixed platelets (1 × 108/mL), ristocetin (1 mg/mL), and Hepes buffer or the inhibitor at various concentrations for 30 minutes at 22°C. Bound ligand was separated from free ligand by centrifugation. And then platelet-associated radioactivity was determined as described elsewhere.2 vWF was iodinated with 125I (Amersham, Arlington Heights, IL) using Iodo-Gen (Pierce Chemical Co, Rockford, IL) according to a method described elsewhere.26
Recognition site(s) of inhibitors were identified in enzyme-linked immunosorbent assay (ELISA) as described elsewhere.23 In brief, polystyrene microtiter plates were coated with a solution of purified vWF or proteolytic fragments of vWF. Purified IgGs were added to the wells coated with vWF or proteolytic fragments of vWF. Then HRP-conjugated goat Igs to either human IgG were added to each well. After incubation, the peroxidase substrate (o-phenylenediamine) was added. The reaction was blocked with 2 mol/L H2SO4.
DDAVP (desmopressin, Minirin, Ferring-Malmo, Sweden) at a dose of 0.3 μg/kg body weight was administered intravenously over 30 minutes. Blood samples were obtained before the infusion and after various time intervals (60, 120, 240, and 480 minutes).
Statistical analysis.
We calculated the specificity, sensitivity, and positive and negative predictive values of inhibitors as a predictor of bleeding.
RESULTS
Of 260 patients enrolled, AvWS was diagnosed in 25 patients (13 women and 12 men; 9.6 %) with a mean age of 54 years (range, 30 to 85 years). Of these 25 patients, 14 had chronic myeloproliferative disorders (CMPD; [CML, 8 patients; PV, 3 patients; ET, 3 patients]), 4 had acute leukemia (AML, 2 patients; AMMoL, one patient; ALL, one patient), 3 had MM, 3 had CLL, and 1 had NHL (Table 1).
Clinical manifestation of AvWS included purpura (in 28% of the patients), petechiae (in 12%), and gastrointestinal bleeding (in 4%). In patients with CMPD, only two patients (14%) had a bleeding complication when AvWS was diagnosed. In contrast, in other groups, 5 out of 11 patients (46%) had bleeding episodes (Table 2).
In patients with CMPD and NHL, the laboratory features were mostly characterized by a prolonged bleeding time, normal vWF:Ag, and a mild or moderate decrease of RIPA and/or vWF:RCoF. In other groups of patients, RIPA and/or vWF:RCoF were moderately or severely impaired, and bleeding time was prolonged in 16 out of 20 patients (80%) (Table 2). The patients' plasmas contained no inhibitory activity against FVIII:C. In some family members of these patients evaluated, vWF:Ag, vWF:RcoF, and RIPA were all normal (data not shown). ABO blood type in these patients was also evaluated because of its major genetic determinant of plasma vWF antigen levels. The normal range for persons with type O is reported 20% to 30% lower than that of other ABO blood types.27 28 In patients with type O in this study, vWF:Ag levels were 7%, 27%, and 32%, suggesting that low levels of vWF:Ag in these patients did not depend on ABO blood types (Table 2).
Multimeric analysis of vWF was done in 6 patients with inhibitor and 6 patients without inhibitor (Table 3). It showed selective loss of large multimers in 11 patients out of 12 patients. This pattern was similar to that of congenital type-2 vWD. Normal multimeric pattern with decrease of vWF:Ag was found only in one patient. Furthermore, 10 out of 13 patients without multimeric analysis showed a relative reduced ratio of vWF:RCoF as compared with vWF:Ag (Table 2).
Mixing studies were evaluated in 24 out of 25 patients (Case 23 was not done). In 8 patients out of 24 patients (33%), the significant reduction of vWF:RCoF activity was observed in the mixture of one volume of normal pool plasma with one volume of patient plasma diluted by eight volumes of saline buffer (vWF:RCoF activity; 10% to 26%) as compared with controls (vWF:RCoF activity; 58%). These results suggested the presence of inhibitor to vWF in these 8 patients (all 3 patients tested with MM, 2 patients with CLL, 1 patient with AML, 1 with CML, and 1 with PV) (Table 2).The inhibitors were of the IgG class and subclasses were IgG1 (7 cases) and IgG2 (1 case). The IgGs from these patients also inhibited the binding of 125I-vWF to platelets in the presence of ristocetin. The recognition site(s) of these inhibitors were then evaluated (Table4). All 6 IgGs evaluated here reacted with native vWF, 5 recognized the 39/34-kD fragment and Fragment III, and 1 recognized Fragment I.
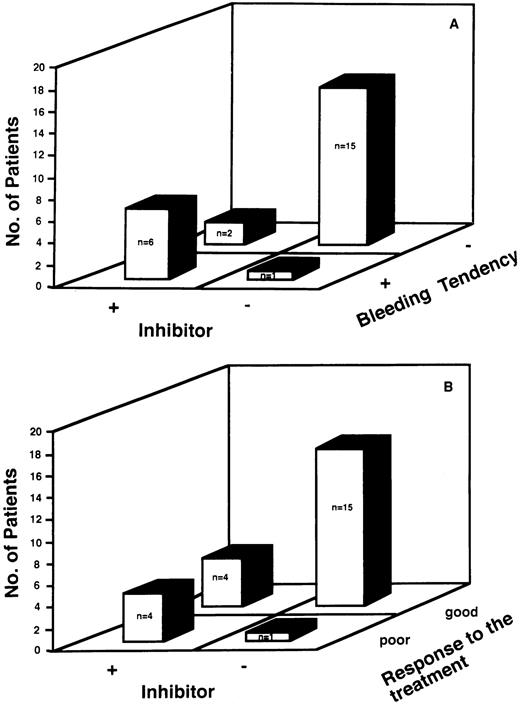
We evaluated the relationship between the presence of inhibitors to vWF and bleeding tendency. There was a close relationship between the presence of inhibitors to vWF and bleeding tendency (Fig 1A). Of the 8 patients with inhibitors, 6 patients (75%) had a bleeding tendency, as did 1 of the 16 patients without inhibitors (6%). The sensitivity of the presence of the inhibitor as a predictor of bleeding was 86%, and the specificity was 88%, with a positive predictive value of 75% and a negative predictive value of 93%.
(A) Relationship between the presence of inhibitors and the clinical manifestation of bleeding tendency. There was a close relationship between the presence of inhibitor to vWF and bleeding tendency. Six patients out of the 8 patients with inhibitors (75 %) had bleeding tendency, as well as one of the 15 patients without inhibitors (6%). The sensitivity of the presence of the inhibitor as a predictor of bleeding was 86%, and the specificity was 88%, with a positive predictive value of 75% and a negative predictive value of 93%. (B) Relationship between the absence of inhibitors and the efficacy of the treatment of underlying diseases and/or DDAVP therapy. Four patients of the 8 patients with inhibitors (50%) had poor response to treatment of the underlying disease and/or DDAVP therapy, as well as one of the 16 patients without inhibitors (6%) . The sensitivity of the absence of inhibitor as a predictor of response to these therapy was 79%, and the specificity was 80%, with positive predictive value of 94% and a negative predictive value of 50%.
(A) Relationship between the presence of inhibitors and the clinical manifestation of bleeding tendency. There was a close relationship between the presence of inhibitor to vWF and bleeding tendency. Six patients out of the 8 patients with inhibitors (75 %) had bleeding tendency, as well as one of the 15 patients without inhibitors (6%). The sensitivity of the presence of the inhibitor as a predictor of bleeding was 86%, and the specificity was 88%, with a positive predictive value of 75% and a negative predictive value of 93%. (B) Relationship between the absence of inhibitors and the efficacy of the treatment of underlying diseases and/or DDAVP therapy. Four patients of the 8 patients with inhibitors (50%) had poor response to treatment of the underlying disease and/or DDAVP therapy, as well as one of the 16 patients without inhibitors (6%) . The sensitivity of the absence of inhibitor as a predictor of response to these therapy was 79%, and the specificity was 80%, with positive predictive value of 94% and a negative predictive value of 50%.
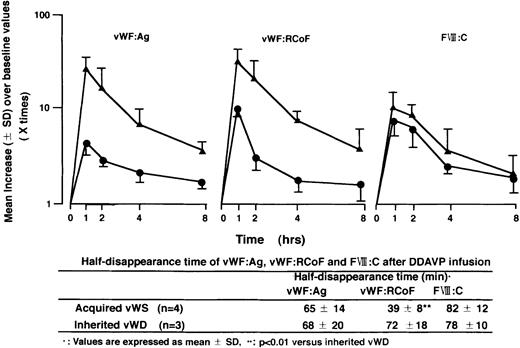
Furthermore, we studied the presence of inhibitors as a possible predictor of the response to treatment of the underlying disease and/or therapy with DDAVP for bleeding. There was a close relationship between the absence of inhibitor to vWF and efficacy of these therapies (Fig 1B). In 20 out of 25 patients (80%), laboratory features of AvWS returned to normal after the treatment of the underlying diseases and/or therapy with DDAVP. Then we studied quantitative changes of vWF:Ag and vWF:RCoF at various periods after DDAVP infusion in 4 patients with AvWS (Case No 2, 8-10), and in 3 patients with inherited type-1 vWD. Although therapy with DDAVP rapidly increased vWF:Ag, vWF:RCoF, and FVIII:C, there was a rapid return of vWF:RCoF to baseline in the patients with AvWS compared with that with inherited vWD, as shown by their half-disappearance time (Fig 2). These results suggested that therapy of DDAVP was transient and that normal return of laboratory features of AvWS mainly depends on the treatment of the underlying diseases. Four patients of the 8 patients with inhibitors (50%) had poor response to the treatment of the underlying disease and/or therapy with DDAVP, as well as one of the 16 patients without inhibitors (6%). The sensitivity of the absence of inhibitor as a predictor of response to this therapy was 79%, and the specificity was 80%, with positive predictive value of 94% and a negative predictive value of 50%.
Quantitative changes of vWF:Ag, vWF:RCoF, and Factor VIII after DDAVP infusion and half disappearance time of these parameters. Results are shown as mean values (±SD) over baseline values taken as 1.
Quantitative changes of vWF:Ag, vWF:RCoF, and Factor VIII after DDAVP infusion and half disappearance time of these parameters. Results are shown as mean values (±SD) over baseline values taken as 1.
In one case (Case No 19), intravenous immunoglobulins were used to correct hemostatic defects because a patient had severe gastrointestinal bleeding. Bleeding time was 15.5 minutes, VWF:Ag, vWF:RCoF, and FVIII:C activity were 28%, 5%, and 6%, respectively. Therapy with DDAVP was not effective to improve hemostatic defects. After the infusion of intravenous immunoglobulins (Venilon, Teijin Co Ltd, Tokyo, Japan) at a dose of 0.3 g/kg for 3 days, levels of VWF:Ag, vWF:RcoF, and FVIII:C activity rapidly increased, reaching normal values by the fourth day. Active bleeding resolved rapidly after immunoglobulin therapy. VWF:Ag, vWF:RCoF, and FVIII:C activity increased up to 150%, 64%, and 58%, respectively. Then these activities gradually decreased, with a return of pretreatment values within 7 days after the last immunoglobulin infusion.
DISCUSSION
AvWS is an uncommon bleeding disorder that has remained difficult to characterize pathophysiologically and challenging to treat successfully. Although the mechanism of AvWS is unclear, several mechanisms may lead to plasma deficiency of vWF. The mechanism involves a decrease in synthesis or a defective release of vWF. However, it seems unlikely that such mechanisms are operative in AvWS because vWF levels increase after DDAVP infusion. The second mechanism involves all kinds of conditions basically affecting the survival of vWF in the circulation. Two general mechanisms were proposed to explain the pathogenesis of AvWS. First, autoantibodies inactivate the biological activities of vWF and induce rapid clearance of vWF from the circulation.5-8 Second, there is a selective absorption of vWF on clonal lymphoid cells in patients with associated lymphoid malignancies.9 With respect to underlying disorders, AvWS is most commonly associated with lymphoproliferative diseases and monoclonal gammopathy, and less often with specific malignancies including chronic myeloproliferative disorders, Wilms' tumor, and adrenal adenocarcinoma.29 AvWS has been infrequently reported in association with autoimmune diseases such as systemic lupus erythematosus.
The sensitivity and specificity of the current standard diagnostic tests such as vWF:Ag, vWF:RCoF, FVIII:C, and bleeding times may be as low as 60%.30 Additional variety is contributed by blood group.27 28 Mean vWF:Ag levels can vary from 70% to 80% for blood type-O individuals to 123% for type AB, when compared with the standard donor plasma pool. As a result, the diagnosis of AvWS may be more readily established in patients of blood type O. In our study, vWF:Ag levels were significantly low in our patients with blood type O, suggesting these patients could be definitely diagnosed as AvWS.
Type-2 congenital vWD is an example of selective loss of large multimers.31 In the majority of the patients with AvWS, the multimeric analysis of plasma vWF showed a type-2 deficiency that is most accurately reflected in vitro by a relative reduced ratio of vWF:RCoF as compared with vWF:Ag in agreement with previous reports.29 32 Our results also showed that 11 out of 12 patients had loss of large molecular weight multimers. In patients with AvWS, vWF is rapidly cleared from the circulation by the evidence of a rapid return of vWF:RCoF to baseline after DDAVP infusion. These results support that one of the mechanisms responsible for development of the syndrome is a rapid clearance of vWF from the circulation independent of the presence of inhibitors
Acquired bleeding disorders are in general often associated with development of an inhibitor, typically an antibody to the functional domain of a coagulation factor. Our study indicated that 8 out of 25 patients (32%) had inhibitors to vWF. All inhibitors tested here were IgGs that reacted with native vWF subunits. Five recognized the 39/34-kD fragment and Fragment III of vWF, which are well-known to retain the binding domain to GPIb.24 25 These results were consistent with the hypothesis that these inhibitors recognized the crucial vWF binding domain(s) to GPIb. Furthermore, it should be noted that there was a close relationship between the inhibitors and bleeding tendency.
Treatment of AvWS depends on the underlying disease process and on the mechanisms responsible for development of the syndrome. Our results also support that treatment in AvWS should, of course, start with treatment of the underlying diseases. Similar to treatment for congenital vWD, patients with AvWS were treated with high doses of cryoprecipitate and this approach was reported to be useful in some patients with AvWS associated with myeloproliferative syndromes.33 However, bleeding was usually controlled in the short term.34 Furthermore, we should consider that factor replacement would be costly and expose the patient to the risk of transfusion-transmitted diseases. DDAVP has been used with some success in AvWS associated with lymphoproliferative and autoimmune disorders, in which the presence of circulating inhibitor to vWF has been shown.35 DDAVP often augments the plasma level of vWF:Ag and vWF:RCoF and corrects the prolonged bleeding time. However, in comparison to patients with congenital vWD, these increased levels and activities are less, vWF cleared more rapidly from the plasma, and the bleeding times were corrected for a short time6 in agreement with our results. On the basis of these results DDAVP may be effective, but not enough for treatment of bleeding episodes in AvWS.
Clinical bleeding tendency was sometimes uncontrollable even when therapy with DDAVP and/or the treatment of the underlying diseases was done in most patients with the inhibitors to vWF. We believe that these patients should immediately receive additional treatment for AvWS. The usefulness of high-dose intravenous immunoglobulins has been shown in patients with antibodies to FVIII:C.36 Recently, possible use for intravenous immunoglobulins in AvWS was shown by in vitro experiments.37 There have been many reports of successful intravenous immunoglobulin infusions for AvWS associated with monoclonal gammopathies of uncertain significance resulting in a rapid and sustained response.38-41 Epistaxis, gastrointestinal hemorrhage, and other active bleeding usually resolve rapidly after intravenous immunoglobulin infusions. In our patient with an inhibitor, severe gastrointestinal bleeding was successively under control by use of immunoglobulin therapy. The mechanism is unknown but might be related to an effect of intravenous immunoglobulin on the clearance of vWF-antibody complex in AvWS.42 It is true that therapy with DDAVP and/or the treatment of the underlying diseases would be the first choice of treatment for bleeding problems in AvWS. The high cost of intravenous immunoglobulin preparation precludes their systematic use in all patients with AvWS. However, they should be reserved for patients with severe bleeding not responding to therapy with DDAVP or for the management of major surgery.
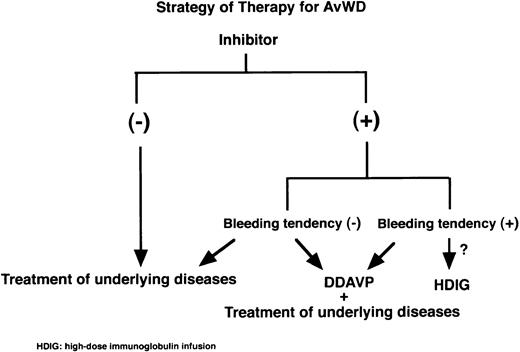
Figure 3 shows one current view of therapy strategy for AvWS. There is no doubt that the treatment of underlying diseases and/or therapy of DDAVP are the first step for treatment of AvWS. When uncontrollable bleeding occurs in cases with inhibitors, intravenous immunoglobulin infusions might be reserved to correct vWF levels.
Strategy of therapy for AvWS. The treatment of underlying diseases and/or DDAVP administration would be the first step for treatment of AvWS. When severe bleeding occurs in AvWS with inhibitors, expensive intravenous immunoglobulin infusions might be considered to correct vWF levels.
Strategy of therapy for AvWS. The treatment of underlying diseases and/or DDAVP administration would be the first step for treatment of AvWS. When severe bleeding occurs in AvWS with inhibitors, expensive intravenous immunoglobulin infusions might be considered to correct vWF levels.
Address reprint requests to Hiroshi Mohri, MD, PhD, Yokohama City University School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama 236, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.