Abstract
Adherence of mature parasitized erythrocytes (PE) of Plasmodium falciparum to microvascular endothelial cells contributes directly to the virulence and pathology of this human malaria. The malarial variant antigen, P falciparum erythrocyte membrane protein 1 (PfEMP1), has been implicated as the PE receptor for CD36 on endothelial cells. We identified the region of PfEMP1 that mediates adherence of PE to CD36 and showed that a recombinant protein fragment from this region blocked and reversed adherence of antigenically different parasites. Sequence variation was evident in the CD36 binding domain of different PfEMP1 genes, yet many highly conserved residues, particularly cysteine residues, are evident. This suggests a highly conserved shape that mediates adherence to CD36. Immunization with the CD36-binding domain elicited sera that are cross-reactive with the different recombinant proteins but are strain-specific for the PE surface. Novel anti-adherence therapeutics and a malaria vaccine may derived from exploitation of the structure of the CD36 binding domain of PfEMP1.
MATURE STAGES of Plasmodium falciparum parasitized erythrocytes (PE) are sequestered from the peripheral circulation by adherence to microvascular endothelium within various organs.1,2 PE adherence is central for both the survival and the pathology of P falciparum.1,3-5 Adherence of P falciparum PE to cerebral blood vessels and consequent local microvascular occlusion contributes directly to the pathology lacking from other human malarias.1 4
Adherence of PE to endothelial cells can be mediated by several molecules on endothelial cells.4,6 CD36 and thrombospondin (TSP) are host cell receptors that support adherence of most P falciparum laboratory strains and patient isolates.4,7-9 Other molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), endothelial leukocyte adhesion molecule-1 (ELAM-1) and chondroitin sulfate mediate adherence of a minority of P falciparum parasites.9-11 Turner et al12 found significant correlation between the expression of ICAM-1 and CD36 in different organs and sequestration of PE. Under flow conditions that mimic in vivo blood flow, only CD36 supports stable stationary adherence of PE, while ICAM-1 mediates PE rolling and adherence to TSP appears to be unstable.13 PE taken from placenta of pregnant women do not adhere to CD36 whereas PE taken from the microvasculature adhere to CD36.14 Thus, CD36 appears to be the critical receptor for anchoring of PE to the microvascular endothelium.
In P falciparum-infected Aotus15 and Saimiri monkeys,16 passive transfer of strain or isolate-specific antimalarial antibodies flushed sequestered PE into the peripheral blood where they were rapidly destroyed. Thus, anti-adherence compounds such as antibodies15,16 or competitive ligands17 could lead to reversal of microvascular obstruction and rapid treatment of acute human cerebral malaria.
Several molecules on the surface of PE have been suggested to participate in PE adherence, including Ag332,18 sequestrin,19 modified band 3,20 and PfEMP1.4,21-24 Numerous studies point to PfEMP1, which is also the malarial surface variant antigen, as the major PE adherence receptor.4 21-26
Recently, we showed that PfEMP1 extracted from PE is an adherence receptor and showed that tryptic fragments of PfEMP1 cleaved from the PE surface specifically bound to CD36, TSP, and ICAM-1.23 PfEMP1 proteins are encoded by the large and diverse var gene family, several members of which have been recently cloned.22,24,27 Several cystein-rich domains in var genes show homology to other malarial proteins involved in attachment and invasion of merozoites including EBA-175 and ebl-1 of P falciparum and the erythrocyte binding protein of P knowlesi and P vivax which bind to the Duffy blood group antigen. These regions named Duffy-binding like (DBL) domains group these malarial receptors and var genes into the DBL superfamily.27
Antibodies against a recombinant protein, rC1-2, corresponding to amino acids 576-808 in the sequence of Malayan Camp (MC) PfEMP1 genes (MCvar-1 and MCvar-2), blocked adherence of MC strain PE to CD36.22 Anti rC1-2 sera also immunoprecipitated the same tryptic fragment of PfEMP1 that was affinity purified with immobilized CD36.23 These results suggested that this domain of the MC PfEMP1 protein may mediate adherence to CD36. The anti-CD36 monoclonal antibodies (MoAbs), OKM5 and 8A6 block PE adhesion to CD36 regardless of antigenic differences of the expressed PfEMP1s.8 28 This suggests that all PE bind to a single region on CD36. Thus, the CD36-binding domains of different (CD36-adherent) PE are expected to have high sequence homology and structural conservation.
In this report we show that the recombinant protein rC1-2 mediates adherence of PE to CD36 and that, in vitro, rC1-2 can block and reverse PE adherence of several P falciparum strains. High structural conservation of corresponding domains of var genes of different P falciparum parasites was also identified. Identification of this CD36-binding domain of PfEMP1 may lead to development of a malaria vaccine and novel anti-adherence therapeutics.
MATERIALS AND METHODS
Parasites. The Malayan Camp MC K+R+C+ (knob,-rosette and adherence-positive) line, denoted MC K+ and other clones and strains of P falciparum were maintained in culture with O+ erythrocytes.22 DNA or RNA were also extracted from the following P falciparum parasites: MC R−, FVO, ItG2-ICAM, ItG2-F6, ItG2-G1 and FCR3-C5 all K+ C+, HB3 (K+ C+, TSP only), Palo Alto (PA) K−C+, FCQ-27 D10 (K+ C+, low-binding TSP only), Dd2 K− (low C+), FCR3-C6 K− C− and MC K−C−. Unless specified all C+ parasites bound to CD36 and TSP. ItG-ICAM and ItG2-F6 also bound to ICAM-1.
Preparation of nucleic acids.P falciparum genomic DNA (gDNA) and total RNA were isolated from mature and late ring stage PE.29,30 The MC K+ and the FVO cDNA libraries were prepared as described previously.22,31 RT-PCR was performed29 with primer 179-3′ (GAGCGGGCGACACTTCTATCT) carrying an EcoRI site followed by polymerase chain reaction (PCR) with primers 179-3′ and 179-5′ (AAGGAAGACAAAATTATGTCCTAT) carrying a BamH1 site.22 Reverse transcriptase-PCR was also performed as described above using the degenerate oligonucleotides (equal ratio) Uni179-5′ (TTTTTTTGG(G/A)(A/T)(G/T)TGGGT(A/T)(T/A/C) (A/C)(T/C)(G/C)A(T/A)ATGTTA) and Uni179-3′ (AC(C/T)AC AATTGATAAA(T/A)T(A/G)CT(A/C)(C/A)A(A/T)CACGAA). Ade- quate removal of contaminating DNA was verified by mock RT-PCR reaction (no enzyme) and PCR across an intron of EBA-175. PCR from gDNA and from cDNA libraries was performed by standard methods.22 The PCR products were digested with BamH1 and EcoRI, cloned into the BamH1-EcoRI sites of the pGEX-3X vector (Pharmacia Biotech Inc, Piscataway, NJ) and expressed as glutathione-S-transferase (GST)-fusion proteins.
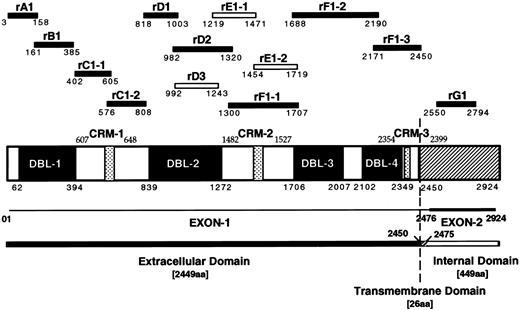
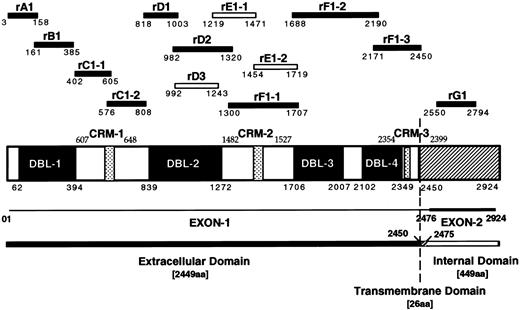
Preparation of recombinant proteins and mutant forms of rC1-2. GST fusion proteins were constructed, expressed in Escherichia coli and purified as described.22 The fragments of PfEMP1 expressed in each fusion protein are identified by their amino acid boundaries (Fig 1 and ref 22). The GST-fusion proteins rA1(3-158), rB1(161-385), rC1-1(402-605), rC1-2(576-808), rD1(818-1003), rD2(982-1320), rF1-1(1300-1707), rF1-2(1688-2190), rF1-3(2171-2450), and rG1(2550-2794) were derived from the sequence of MCvar-1.22 rD3(992-1243), rE1-1(1219-1471), and rE1-2(1454-1719) were specific to the MCvar-2 PfEMP1.22 The rF1-2 GST-fusion protein was not stable and was tested as a Maltose binding protein (MBP) fusion only. GST-fusion proteins representing different fragments of the 233 amino acids rC1-2 GST fusion, denoted rC1-2 [1-233], were generated by PCR as above and are indicated with the corresponding boundaries of rC1-2 [1-233]. Cysteines at various positions were replaced by using a primer with a serine codon in place of the corresponding cysteine residue. MBP-fusion proteins were generated as above using a modified pMAL vector compatible with the BamH1-EcoRI cloning sites. The GST-fusion rGA62-5 derived from clone A62 was described earlier.22
Schematic diagram of the MC PfEMP1 protein and recombinant protein fragments. Schematic drawing showing the four DBL domains and the three cysteine rich motifs (CRM) of the MCvar-1 PfEMP1 gene with amino acid boundaries. Recombinant proteins (GST or MBP fusion) derived from the MCvar-1 sequence (filled bars) and fragments specific for the MCvar-2 sequence (open bars) are shown.
Schematic diagram of the MC PfEMP1 protein and recombinant protein fragments. Schematic drawing showing the four DBL domains and the three cysteine rich motifs (CRM) of the MCvar-1 PfEMP1 gene with amino acid boundaries. Recombinant proteins (GST or MBP fusion) derived from the MCvar-1 sequence (filled bars) and fragments specific for the MCvar-2 sequence (open bars) are shown.
Antibodies and soluble receptors. Mouse MoAb 179 (Affymax Research Institute) recognizes an epitope incorporated into the carboxyl terminus of host receptors expressed as phosphoinositol glycan-linked extracellular domains. Mouse MoAb 141 (Affymax Research Institute) recognizes GST. Preparation of soluble host receptors, CD36, ICAM-1, VCAM-1, E-selectin, P-selectin, and L-selectin, was described earlier.22,23 Antibodies to recombinant proteins of PfEMP1 were prepared as described before.22
Cytoadherence microassay and binding of CHO-CD36 cells to rC1-2. Adherence of PE to cells or immobilized proteins was performed by standard methods.8,22 Blockade of PE adherence by recombinant protein was tested by preincubating the recombinant protein with CD36 immobilized to plastic via MoAb 179, 1 hour 21°C, before addition of PE in binding media (BM, RPMI-1640, 25 mmol/L HEPES, 1% bovine serum albumin [BSA] pH 6.8) containing recombinant protein. Reversal of adherence was performed by binding of PE to CD36 for 30 minutes, nonadherent cells were washed away with binding media (BM) and the bound PE were incubated with BM containing antibodies or recombinant protein up to 45 minutes. For binding of CHO-CD36 cells to rC1-2, GST-fusion proteins were immobilized on petri dishes coated, 1 hour 21°C, with 7 μL of 50 μg/mL MoAb 141. Fifty microliters of 2 × 106 cells/mL in BM were added, 1 hour at 37°C, washed four times with BM, fixed, stained, and counted.22
Affinity-purification of CD36 with immobilized recombinant fragments of PfEMP1. Twenty-five microliters of GammaBind Plus Sepharose beads (Pharmacia Biotech Inc) was precoated, 90 minutes 21°C, with 15 μg of MoAb 141 (anti GST), then incubated as described23 with 5 μg of recombinant protein or with 50 μL of bacterial lysate containing comparable amounts (5 to 15 μg) of recombinant protein. 0.5 mL of soluble receptor at 0.5 to 1 μg/mL in BM, expressing a sequence recognized by MoAb 179 was added and incubated, 2 hours 21°C with the beads. The beads were washed twice with BM, once with BM without BSA and solubilized in 40 μL of sodium dodecyl sulfate (SDS) sample buffer. Five microliter samples were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) immunoblotted and probed with a 1:5,000 dilution of biotinylated MoAb 179 as primary Ab followed with peroxidase-conjugated streptavidin (Jackson Immunoresearch Inc, West Grove, PA) at 1:5,000 dilution.22
RESULTS
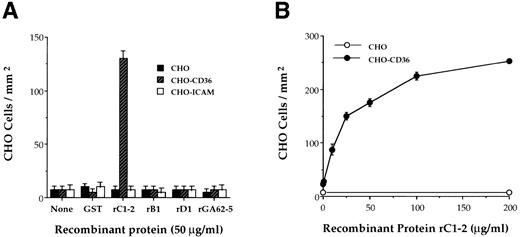
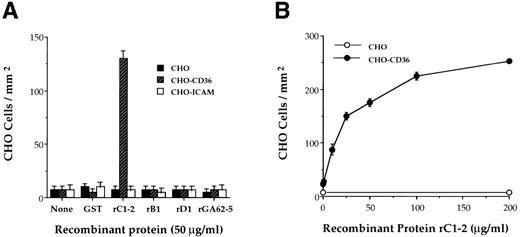
rC1-2 specifically binds CD36. Antibodies against the recombinant protein, rC1-2, corresponding to amino acids 576-808 of the MC PfEMP1 genes MCvar-1 and MCvar-2 (Fig 1), were shown to block adherence of MC strain PE to CD36.22 These antibodies also immunoprecipitated a tryptic fragment derived from PfEMP1 affinity purified with immobilized CD36.23 To determine directly whether this region of PfEMP1 is involved in PE adherence to CD36, the rC1-2 GST fusion protein, expressed in E coli, was tested for binding to CD36. rC1-2 immobilized on plastic promoted the binding of Chinese hamster ovary (CHO) transfected cells expressing CD36 (CHO-CD36) but not of CHO or CHO-ICAM-1 transformants (Fig 2A). None of the other recombinants tested, including recombinants derived from adjacent regions of the MC PfEMP1 (rB1 and rD1), nor GST alone, mediated adherence of CHO-CD36 cells (Fig 2A). Adherence of CHO-CD36 cells to rC1-2 was significantly greater than that of CHO cells over the entire range of rC1-2 concentrations tested (Fig 2B). The interaction of CD36 with PfEMP1 was specific for the rC1-2 recombinant protein (Fig 3A). None of the other recombinant protein from the MC PfEMP1 (Fig 1), nor GST per se, bound CD36 (Fig 3A). Identical results were obtained with recombinant proteins expressed as MBP fusion proteins (data not shown). The rC1-2 fragment itself expressed in yeast also bound CD36 (Table 1). The binding properties of rC1-2 were specific for CD36 (Fig 3B). ICAM-1, VCAM-1, and ELAM-1 that mediate adherence of some parasite strains10 but not the MC strain22 were not affinity purified with beads bearing rC1-2 (Fig 3B). No binding of rC1-2 to TSP was detected (data not shown), in agreement with our earlier observations that the CD36 and TSP binding sites in MC strain PfEMP1 reside on different tryptic fragments cleaved from the PE surface.23
Binding of CHO-CD36 cells to the rC1-2 fragment of MC PfEMP1. GST-fusion proteins derived from the MC PfEMP1 sequence were immobilized on petri dishes and incubated with 50 μL of 2 × 106 cells/mL of CHO, CHO-CD36, and CHO-ICAM-1 cells. Results are presented as number of bound cells/mm2 ± STD. (A) CHO-CD36 cells specifically bind to immobilized rC1-2 but not to rB1 and rD1 recombinant proteins from MC PfEMP1, rGA62-5, GST, or to the immobilizing MoAb 141 alone (none). CHO cells or CHO-ICAM-1 cells did not bind to any of the recombinant proteins tested. (B) Concentration-dependent binding of CHO-CD36 cells (•) but not CHO cells (○) to immobilized rC1-2.
Binding of CHO-CD36 cells to the rC1-2 fragment of MC PfEMP1. GST-fusion proteins derived from the MC PfEMP1 sequence were immobilized on petri dishes and incubated with 50 μL of 2 × 106 cells/mL of CHO, CHO-CD36, and CHO-ICAM-1 cells. Results are presented as number of bound cells/mm2 ± STD. (A) CHO-CD36 cells specifically bind to immobilized rC1-2 but not to rB1 and rD1 recombinant proteins from MC PfEMP1, rGA62-5, GST, or to the immobilizing MoAb 141 alone (none). CHO cells or CHO-ICAM-1 cells did not bind to any of the recombinant proteins tested. (B) Concentration-dependent binding of CHO-CD36 cells (•) but not CHO cells (○) to immobilized rC1-2.
Affinity purification of CD36 with immobilized rC1-2 and fragments of rC1-2 [1-233]. Soluble receptors were incubated with bead-immobilized GST fusion recombinant proteins derived from the sequence of MC PfEMP1. Binding of CD36 and other host receptors was detected by Western blotting. (A) CD36 specifically binds to immobilized rC1-2 and not to GST or to other GST-fusion proteins (Fig 1) from the MC PfEMP1. rF1-2 was tested as an MBP-fusion protein. (B) Immobilized rC1-2 specifically binds CD36 and not other host cell receptors: P-Selectin, E-Selectin (ELAM-1), VCAM-1, and ICAM-1. These receptors do not mediate adherence of MC strain PE. (C) Deletion fragments of rC1-2 [1-233] were amplified by PCR with the appropriate primers, expressed in E coli and immobilized on Ab coated beads directly from 50 μL of bacterial lysate. CD36 binds to the 233 amino acid rC1-2 fragment of MC PfEMP1, denoted rC1-2 [1-233], and to a fragment representing a 41 amino acid deletion from the carboxy terminus, denoted rC1-2 [1-192]. Other fragments of rC1-2, including deletions from the amino or the carboxy end of rC1-2 and mutant forms in which cysteine residues were replaced with serine were also tested. These results are summarized in Table 1.
Affinity purification of CD36 with immobilized rC1-2 and fragments of rC1-2 [1-233]. Soluble receptors were incubated with bead-immobilized GST fusion recombinant proteins derived from the sequence of MC PfEMP1. Binding of CD36 and other host receptors was detected by Western blotting. (A) CD36 specifically binds to immobilized rC1-2 and not to GST or to other GST-fusion proteins (Fig 1) from the MC PfEMP1. rF1-2 was tested as an MBP-fusion protein. (B) Immobilized rC1-2 specifically binds CD36 and not other host cell receptors: P-Selectin, E-Selectin (ELAM-1), VCAM-1, and ICAM-1. These receptors do not mediate adherence of MC strain PE. (C) Deletion fragments of rC1-2 [1-233] were amplified by PCR with the appropriate primers, expressed in E coli and immobilized on Ab coated beads directly from 50 μL of bacterial lysate. CD36 binds to the 233 amino acid rC1-2 fragment of MC PfEMP1, denoted rC1-2 [1-233], and to a fragment representing a 41 amino acid deletion from the carboxy terminus, denoted rC1-2 [1-192]. Other fragments of rC1-2, including deletions from the amino or the carboxy end of rC1-2 and mutant forms in which cysteine residues were replaced with serine were also tested. These results are summarized in Table 1.
We endeavored to identify a smaller fragment of rC1-2 containing the CD36-binding function. Recombinant GST fusion proteins representing different fragments of rC1-2 (hereafter denoted rC1-2 [1-233]) were tested for binding CD36 (Fig 3C and Table 1). Deletion of 54 residues from the carboxyl end of rC1-2, yielding rC1-2 [1-179], had no effect on binding of CD36 (Fig 3C and Table 1). However, deletion of an additional 39 or more residues (Table 1) including two cysteine residues (positions 159 and 168), yielding fragments equal or smaller than rC1-2 [1-140], ablated adherence to CD36 (Fig 3C). Replacement of either or both of these cysteines with serine in rC1-2 [1-233] or rC1-2 [1-179] greatly reduced binding to CD36 (Table 1). Hence, the region 140-179 of rC1-2, and in particular its cysteine residues, is essential for maintaining CD36 binding. Deletion of the first 9 amino acids yielding rC1-2 [10-179] or deletion of larger fragments from the amino end of rC1-2 ablated binding to CD36 (Table 1). Thus, rC1-2 [1-179] is the smallest fragment retaining the CD36 binding property.
rC1-2 contains 7 cysteine residues, 5 of which are located in a cysteine-rich motif (CRM) near the amino end (residues 32-51 in rC1-2) characterized by the sequence CX8CX3 CX3CXC.22 Separate replacement of each of the five cysteines in the CRM with serine resulted in very low expression or a nonfunctional fusion protein, except for rC1-2[1-179] ser49 that had approximately half of the CD36-binding activity of rC1-2 (Table 1). Hence, the amino-terminus of rC1-2 including its CRM, is important but not sufficient for adherence to CD36. Reduction of rC1-2 followed by alkylation with iodoacetamide was associated with lower mobility on SDS-PAGE and lack of binding of CD36 (Table 1). Without alkylation the protein bound CD36 (Table 1). Thus, binding of rC1-2 to CD36 requires a conformation imposed by cysteine residues and is not mediated by linear sequence alone.
rC1-2 blocks and reverses adherence of PE to CD36. Antibodies against rC1-2 blocked adherence of PE from strain MC, but not PE from other strains.22 In contrast, preincubation of immobilized CD36 with rC1-2, followed by addition of MC strain PE, ablated PE adherence to CD36 of all strains tested. Blockade of adherence of several P falciparum strains known to express antigenically and biochemically different forms of PfEMP122 23 is depicted in Fig 4A. Blockade of adherence was observed also with strains MC R−, FCR3-C5, ItG2-F6, and Dd2 (data not shown). GST and other recombinant proteins derived from the sequence of PfEMP1 were without effect (Fig 4A and unpublished data). rC1-2 and other recombinant proteins, expressed in E coli, had no effect on PE adherence to TSP (not shown). The malarial protein fragment itself, cleaved by thrombin from its GST fusion partner, or expressed with a histidine tag in yeast, was also active and blocked PE adherence to CD36 (data not shown). A dose-response analysis of the competitive inhibition of PE binding by rC1-2 (Fig 4B) revealed that rC1-2 [1-233] inhibited adherence of MC strain PE to CD36 by 50% at about 63 μg/mL (IC50∼ 1.2 μmol/L). rC1-2 [1-179] inhibited adherence to CD36 with an IC50 of ∼0.78 μmol/L (Fig 4B). At the maximal concentration tested inhibition was 80% (Fig 3). However, >90% inhibition was observed at 100 μg/mL with several other preparations of rC1-2 [1-179] (data not shown). Such variability may result from different concentrations of correctly folded recombinant proteins in each sample. rC1-2 was also able to reverse PE adherence to CD36 with an IC50 of about 25 μg/mL (Fig 4C). GST had no effect over the concentration range tested (Fig 4B and 4C).
Blockade and reversal of PE adherence to CD36. Petri dishes coated with CD36 were preincubated with recombinant protein before testing for binding of PE. (A) rC1-2 but not other recombinant proteins, at 100 μg/mL, specifically blocked adherence to CD36 of PE from four phenotypically different P falciparum strains and clones. Strain MC R+ (▪); clone ItG2-ICAM (▨); clone ItG2-G1 (); clone Palo Alto (K−C+) (□). None of the other recombinant proteins affected PE adherence to CD36. Other recombinant proteins of MC PfEMP1 (Figs 1 and 2) did not block adherence of MC strain PE to CD36 (not shown). (B) Concentration-dependent blockade of PE adherence (strain MC) to CD36 with rC1-2 and rC1-2 [1-179]. IC50 values were 1.2 μmol/L for rC1-2 and 0.78 μmol/L for rC1-2 [1-179]. (C) Concentration dependent reversal of MC PE adherence to CD36 with rC1-2. Adherent PE were incubated with GST or rC1-2 [1-233] and remaining adherent cells counted. The IC50 value was 0.5 μmol/L.
Blockade and reversal of PE adherence to CD36. Petri dishes coated with CD36 were preincubated with recombinant protein before testing for binding of PE. (A) rC1-2 but not other recombinant proteins, at 100 μg/mL, specifically blocked adherence to CD36 of PE from four phenotypically different P falciparum strains and clones. Strain MC R+ (▪); clone ItG2-ICAM (▨); clone ItG2-G1 (); clone Palo Alto (K−C+) (□). None of the other recombinant proteins affected PE adherence to CD36. Other recombinant proteins of MC PfEMP1 (Figs 1 and 2) did not block adherence of MC strain PE to CD36 (not shown). (B) Concentration-dependent blockade of PE adherence (strain MC) to CD36 with rC1-2 and rC1-2 [1-179]. IC50 values were 1.2 μmol/L for rC1-2 and 0.78 μmol/L for rC1-2 [1-179]. (C) Concentration dependent reversal of MC PE adherence to CD36 with rC1-2. Adherent PE were incubated with GST or rC1-2 [1-233] and remaining adherent cells counted. The IC50 value was 0.5 μmol/L.
The CD36 binding domain of different var genes. The primers used to amplify the rC1-2 [1-179] region of PfEMP1 (179 primers) were specific to P falciparum and did not yield a PCR product from gDNA of several other malaria parasites including P knowlesi, Plasmodium fragile, Plasmodium coatneyi and Plasmodium cynomolgi. PCR products were obtained with the 179 primers from 11 culture-adapted strains and clones of P falciparum. These sequences can be grouped into three distinct categories characterized by >95% identity between the different members of each group (first three sequences in Fig 5). The “MC group” includes all the MC sequences including the expressed sequence (MCvar-1) of MC R+ (cDNA and mRNA sequences) and sequences originated from gDNA of all MC parasites (MC R+, MC R− and the nonadherent MC K− ). The “ItG/FVO group” of sequences is represented by the cDNA sequence of clone FVO, RT-PCR product of ItG-ICAM, and gDNA sequences from ItG-ICAM, ItG2-F6, ItG2-G1, FCR3-C5, FCR3-C6, and Palo Alto parasites. The third type, denoted “HB3,” includes the RT-PCR products of ItG2-F6 and FCR3-C5 parasites and the gDNA and cDNA products of HB3. Degenerate universal primers (uni179) were deduced from the above sequences. Fourteen different sequences depicted in Fig 5, were obtained by RT-PCR from mRNA of seven P falciparum strains (MC R+, MC R−, ItG-ICAM, FVO, HB3, PA, and D10) and two from gDNA of strain Dd2 (var-6 and the var gene Dd2 var-7 like, homologous to the expressed Dd2var-7 ). Similar sequences were found in gDNA of 11 P falciparum strains tested. Several of the sequences, such as the Dd2 var-7 like and the ItG/FVO type, were apparent in more than one strain and more that one of the above sequences were represented in gDNA from different strains. However, none of the genomes of individual strains carried all 16 sequences (not shown). As already published,22,24 27 we found expression of more than a single var gene among the PE of a particular strain (Fig 5). PCR products with similar size were observed from 20 wild isolates and were positive for hybridization with DNA corresponding to rC1-2 [27-80] (D. Baruch, unpublished data). This region of PfEMP1 is therefore represented and size-conserved among geographically diverse P falciparum parasites.
Alignment of the predicted amino acid sequences of the corresponding CD36 binding domain of different var genes. The rC1-2 [1-179] and the rC1-2 [10-151] sequences were amplified by RT-PCR from the expressed var genes of different P falciparum strains and clones (except for the gDNA clone Dd2 var-6). These sequences were detected in gDNA of many strains. Several sequences appeared as expressed var genes (by RT-PCR) in more than one strain. The MC type (first sequence) includes sequences (cDNA, gDNA and RT-PCR) originated from MC parasites (R+, R−, and K−). The ItG/FVO type (second sequence) represents sequences from cDNA of clone FVO, RT-PCR product of ItG-ICAM and gDNA sequences from ItG-ICAM, ItG2-F6, ItG2-G1, FCR3-C5, FCR3-C6, and Palo Alto parasites. The HB3 type (third sequence) includes the RT-PCR products of ItG2-F6 and FCR3-C5 and the gDNA and RT-PCR products of HB3. Other depicted sequences represents expressed var genes (with the exception of Dd2 var-6) amplified with the Uni179 primers from the indicated strains. The emerging consensus sequence of conserved amino acids is depicted on top of the alignment. Highly conserved amino acids are indicated in red, conserved substitutions are indicated by orange, and nonconserved substitutions by green. The conserved cysteine residues are marked by asterisks and additional cysteine residues are shown in blue. These sequence data are available from GenBank under accession numbers AF008978-AF009001.
Alignment of the predicted amino acid sequences of the corresponding CD36 binding domain of different var genes. The rC1-2 [1-179] and the rC1-2 [10-151] sequences were amplified by RT-PCR from the expressed var genes of different P falciparum strains and clones (except for the gDNA clone Dd2 var-6). These sequences were detected in gDNA of many strains. Several sequences appeared as expressed var genes (by RT-PCR) in more than one strain. The MC type (first sequence) includes sequences (cDNA, gDNA and RT-PCR) originated from MC parasites (R+, R−, and K−). The ItG/FVO type (second sequence) represents sequences from cDNA of clone FVO, RT-PCR product of ItG-ICAM and gDNA sequences from ItG-ICAM, ItG2-F6, ItG2-G1, FCR3-C5, FCR3-C6, and Palo Alto parasites. The HB3 type (third sequence) includes the RT-PCR products of ItG2-F6 and FCR3-C5 and the gDNA and RT-PCR products of HB3. Other depicted sequences represents expressed var genes (with the exception of Dd2 var-6) amplified with the Uni179 primers from the indicated strains. The emerging consensus sequence of conserved amino acids is depicted on top of the alignment. Highly conserved amino acids are indicated in red, conserved substitutions are indicated by orange, and nonconserved substitutions by green. The conserved cysteine residues are marked by asterisks and additional cysteine residues are shown in blue. These sequence data are available from GenBank under accession numbers AF008978-AF009001.
Alignment of the predicted amino acid sequences revealed relatively high sequence and potential structural conservation (Fig 5). The homology of the sixteen rC1-2 sequences exceeded any known homology between exon-1 sequences of var genes. Such homology is suggestive of a highly conserved function for this region of PfEMP1.
The cysteines of the rC1-2 [1-179] fragment, in particularly the 5 cysteines within cysteine rich motif 1 (CX8CX3CX3CXC) are highly conserved among the different var genes (Fig 5). This motif has the consensus sequence of C(I/L)XNX4-7CX3-5 C(N/K)XXCXCF. These observations are compatible with the important role of the cysteines in the structure-function of the CD36-binding domain. Apart from the CRM motif we note 5 additional regions of homology (Fig 5). The first homology region, residues 10-26 shows very high homology and has the consensus sequence FFWXWVXXMLXDS(L/I/V)XWR. The second homology domain, residues 51-85 includes part of the CRM-1 motif with C(N/K)XXCXCFXKWNXXKXXEWXXIKXHFXKQK(XX)DI as the consensus sequence. The next two domain shows lower homology and has the consensus sequence LEX(V/L)LXKXXLL (residues 107-117) and GX4IX(H/R)XX(L/M)LXXE (residues 126-147). Homology domain number 5 is very conserved with the consensus sequence of TTIDKXLXHE (residues 179-188). It is interesting to note that although the first 9 residues are not conserved they were important for the correct folding of the protein in E coli (Table 1). The high homology observed could also come from the primers used to generate the PCR products that amplify a subset of var genes that has high homology in this region. Examination of available var sequences27 32 revealed high conservation of the CRM and the homology domain at the amino terminus but less or low conservation of other homology domains, particularly domain 5.
GST-fusion proteins corresponding to rC1-2 [1-179] from 11 parasite sequences (shown as MC, FVO, and HB3 type in Fig 5) and from Dd2 var 7, were expressed in E coli and tested for binding to CD36. Each of the three “MC type” sequences (from MC R+, MC R− and MC K−) strongly bound CD36. Seven examples of fusion proteins from the other groups showed binding to CD36; however, binding was significantly lower compared to rC1-2 [1-179] of MC parasites. The Dd2 var-7 and the PA recombinant proteins had no activity at all even when tested at high concentrations (data not shown). Reduced activity or zero activity in these recombinant proteins could derive from multiple factors including incorrect folding of the protein in the bacterial host, absence of essential residues located outside the cloned region, or expression of a non-CD36 binding var gene.
Antibodies against the rC1-2 [1-179] of different P falciparum provided additional support for the role of this region in adherence to CD36. Antibodies to the rC1-2 [1-179] fusion protein of the MC and the ItG (ItG/FVO type, Fig 5) strains agglutinated and blocked adherence of PE expressing the same sequence without affecting PE expressing heterologous sequences (Table 2 and ref 22). Sera to the ItG type rC1-2 [1-179] readily agglutinated FVO PE (in Aotus monkey erythrocytes) and blocked adherence of FVO PE by more than 70% without affecting binding of strain MC PE (Table 2). These antibodies also agglutinated PE of strain ItG, which share the same rC1-2 sequence with FVO (Table 2). We were unable to show that these antibodies also block adherence of strain ItG PE (Table 2). ItG population express several var genes with different rC1-2 [1-179] sequences (Fig 5). Although we assume that the binding of PE expressing the FVO/ItG sequence was blocked the sera had no effect on adherence of PE expressing other rC1-2 [1-179] sequences in the ItG population. Antibodies to the HB3 type did not agglutinate or block adherence of PE from any of the strains tested (Table 2).
Taken together, these studies with the rC1-2 [1-179] region of different var genes support the concept that this region of PfEMP1 mediates adherence of P falciparum PE to CD36.
Reactivity of sera with the CD36-binding domain of different PfEMP1s. Despite the homology between the CD36 binding domains of different var genes, immunization with these domains appears to elicit strain-specific antibodies. Anti rC1-2 [1-179] sera from various animals were found to be strain or sequence specific for both PE agglutination and blockade of adherence (Table 2). However, cross-reactivity was observed frequently when these sera were tested for reactivity with the recombinant protein by ELISA or Western blotting (Table 2). Consistent with these data, many Aotus and human sera (Table 2 and refs 22, 25) and sera generated against different exposed regions of PfEMP122 (and unpublished data, 1995-1996) mediate strain-specific PE agglutination. Furthermore, none of the Aotus sera raised by repeated infection with P falciparum strains reacted with any of the PfEMP1 recombinant proteins on Western blots (D Baruch, unpublished observations). We propose that the immunodominant agglutinating epitopes expressed on the surface of PE are conformation-dependent, strain-specific epitopes and that cross-reactive linear epitopes, evident by enzyme-linked immunosorbent assay (ELISA) and Western blotting, are inaccessible to antibodies on the surface of the infected cell. Hence, the parasite can efficiently evade the host immune system even when cross-reactive antibodies (to linear epitopes) are generated.
None of several potent agglutinating and adherence blocking monkey and human sera (tested individually or pooled) showed high-titer antibodies to the CD36-binding domain (Table 2). These sera also failed to immunoprecipitate the small CD36-binding tryptic fragments of the MC PfEMP1.23 The CD36-adherence blocking activity of these sera is probably due to steric hindrance or to conformational alterations mediated by antibody binding. This is evident by the inability of the monkey anti-FVO sera to agglutinate PE from the ItG strain even though the CD36-binding domains of these strains show sequence identity and anti-ItG rC1-2 [1-179] sera agglutinate PE of both strains (Table 2). Thus, the CD36-binding domain may not elicit high-titer antibodies in P falciparum infected humans and monkeys, but is a very potent immunogen when expressed as a recombinant protein.
DISCUSSION
Sequestration of mature PE from the peripheral circulation plays a major role in the virulence and pathogenesis of P falciparum.1,3-5 Adherence of mature PE to microvascular endothelial cells is a complex process that may involve interactions between multiple receptors.4,10,33 Identification of the adherence molecules and particular domains that mediate PE adherence to different host receptors is an essential step to understand the molecular pathogenesis of P falciparum and for development of anti-adherence therapeutics. Several host receptors, including CD36, TSP, ICAM-1, VCAM-1, ELAM-1, and chondroitin sulfate were shown, in vitro, to promote binding of PE.10,11,28,34,35 Most adherent PE from wild isolates and culture-adapted strains bind to CD36, illustrating its importance as host receptor.7-9 We describe here a fragment from the PfEMP1 protein, corresponding to amino acids 576 to 755 of the MC PfEMP1 gene,22 denoted rC1-2 [1-179], which mediates PE adherence to the major host receptor, CD36. This fragment specifically bound CD36 and also blocked and reversed PE adherence to CD36 with several parasite strains. The specific binding of this region with CD36 and failure of other fragments from PfEMP1 to exhibit CD36 binding is consistent with the concept of independent binding sites for each of the host receptors.23,36 Supplemental evidence supports the role of this fragment in adherence to CD36. First, treatment of intact PE with mild trypsinization releases a fragment derived from PfEMP1 that is affinity purified by immobilized CD36 and is immunoprecipitated by anti rC1-2 antibodies.23 Second, antibodies against the sequence diverse rC1-2 regions from strain MC or ItG/FVO specifically blocked adherence of PE to CD36.
The CD36 binding properties of the rC1-2 fragment appear to be dependent on its conformation. rC1-2 molecules unfolded by reduction and alkylation or mutated by substitution of cysteine residues lose their ability to bind CD36 (Table 1). Of the seven cysteines in the rC1-2 region, only substitution of cysteine 49 rendered a functional protein, indicating that this cysteine may be unpaired.
Antibodies to CD36, such as OKM5 and 8A6, block adhesion of different P falciparum strains,8,28 suggesting that all parasite strains bind to the same adhesion pocket on CD36. Blockade of PE adhesion of different strains by rC1-2 support this observation. Thus, the CD36 binding domain on the variant molecule PfEMP1 is predicted to have highly conserved sequence or three-dimensional structure. In Trypanosomes, the variant surface glycoproteins of Trypanosome brucei are known to have similar three dimensional shape although they display high sequence diversity.37 Much of this structural conservation is attributed to the high conservation of the cysteine residues.37 Examination of the sequences corresponding to rC1-2 in diverse parasites (Fig 5 and ref 27) shows that these sequences are not identical but display relatively high homology, particularly the cysteine residues at both ends of rC1-2. This conservation is predicted to result in highly conserved three-dimensional structure. Several other amino acids depicted on the consensus sequence line also show substantial sequence conservation. We submit that these conserved residues are involved in correct folding of the CD36-binding domain of diverse sequences to generate a conserved shape that binds CD36. This shape, represented by the MC rC1-2 [1-179] recombinant protein, binds to CD36 and can block adherence of PE expressing antigenically distinct PfEMP1 proteins. Thus, the specific conformation of the fragment is likely essential for function.
Antibodies to the CD36 binding domain of different var genes and anti-PfEMP1 antibodies show strain-specific agglutination and blockade of adherence.22 Strain-specific agglutination and blockade of adherence was evident in several experiments with monkey or human sera25,38,39 and anti-PfEMP1 sera.22,40 When tested by ELISA and Western blotting, substantial cross-reactivity was found with the different recombinant proteins (Table 2) and among PfEMP1 of different strains.22 Thus, the linear cross-reactive epitopes recognized by these sera are not readily available on the PE surface. Furthermore, highly agglutinating monkey sera do not react with any recombinant protein of PfEMP1 on Western blots although positive for the PfEMP3 12.1.3 recombinant protein.29,30 These sera recognize some recombinant proteins of PfEMP1 by ELISA and are reactive with PfEMP1 by PE agglutination and immunoprecipitation (Table 2,22,23,41 and D. Baruch, unpublished data). We assume that antibodies that react with the surface of PE recognize predominantly conformation-dependent epitopes instead of linear ones. Thus, sera elicited by immunization with a recombinant protein that are cross-reactive by several immunochemical methods are not necessarily cross-reactive with the surface of the PE. This apparent lack of surface cross-reactivity may also affect antibody reactivity with the surface of merozoites and sporozoites of malaria parasites42 and have general implications on the assessment of antibody reaction with vaccine antigens. We also found that several potent PE agglutinating human and monkey sera do not have detectable high titer antibodies against the CD36 binding domain (ref 23 and unpublished data). It appears that variability within the PfEMP1 sequence has evolved such that the parasite can maintain adherence to CD36 yet evade host immunity at the same time. In contrast, immunization of rats, rabbits, goats, and mice with the CD36 binding domain elicited high titer antibodies. These antibodies were reactive with the surface of the PE in a strain-specific manner (ref 22 and D.I.B., unpublished observations, 1995-1997).
With the identification of this PE CD36-binding domain, vaccine elicitation of anti-adherence immunity and novel therapeutics can be envisioned. The importance of PE adherence to CD36 in the survival and pathogenesis of P falciparum can be directly addressed with new and specific anti-adherence agents, such as antibodies and recombinant proteins that disrupt the CD36-PE interaction. Strain-specific antibodies, specific for the PE surface variant antigens, developed in P falciparum or P knowlesi-infected monkeys protect the animal from challenge with the homologous strain.15,16,43,44 In Aotus and Saimiri monkeys infected with P falciparum, passive transfer of such strain-specific sera disrupted sequestration and led to rapid clearance of the infection.15 16 Thus, anti-PfEMP1 antibodies have the ability, in vivo, to interfere with PE sequestration, conferring rapid clearance and protection from P falciparum infection. It is conceivable that immunization with the CD36 binding domain will produce comparable results. However, sera from infected animals, as well as antibodies elicited by immunization with particular rC1-2 molecules (MC or ItG/FVO group) do not react with the surface of PE of diverse PfEMP1 phenotypes. This strain-specific immunity resulting from the sequence diversity of var genes is a major obstacle for development of an antimalarial vaccine based on PfEMP1. However, several stretches of highly conserved amino acids are depicted among the various sequences of the CD36 binding domain (Fig 5). We also note the apparent lack of high titer antibodies reactive with the CD36 binding domain in human sera from endemic areas (ref 23 and D.I.B., unpublished observations, 1996-1997). Immunization with peptides representing the more conserved regions of this domain may elicit cross-reactive immunity.
In conclusion, a recombinant fragment from PfEMP1 has been shown for the first time to bind a specific host receptor and to block adherence of PE from different P falciparum strains to this protein, CD36. Hence, PfEMP1 is not only a variant antigen on the PE surface but also a receptor responsible for adherence of PE to CD36. Additional studies are required to identify whether an immunogen derived from this functional region of PfEMP1 can elicit antibodies reactive with all CD36-adherent PE. If the shape of PfEMP1 required for binding to CD36 is universally conserved, novel anti-adherence therapeutics can be envisioned in the form of antibodies, recombinant protein, peptides or peptidomimetics that disrupt the CD36-PE interaction.
ACKNOWLEDGMENT
We thank David Kaslow for preparation and expression of recombinant proteins in yeast, Xin-zhuan Su and Tom E. Wellems for the Dd2 var-7 GST-fusion protein.
Supported by Affymax Research Institute and a grant to R.J.H from the United States Agency for International Development Malaria Vaccine Development Program (Washington, DC) (HRN-6001-A-00-2043-00).
Address reprint requests to Dror I. Baruch, PhD, Laboratory of Parasitic Diseases, NIAID, NIH, Bldg 4, Room B1-37, 9000 Rockville Pike, Bethesda, MD 20892.

![Fig. 3. Affinity purification of CD36 with immobilized rC1-2 and fragments of rC1-2 [1-233]. Soluble receptors were incubated with bead-immobilized GST fusion recombinant proteins derived from the sequence of MC PfEMP1. Binding of CD36 and other host receptors was detected by Western blotting. (A) CD36 specifically binds to immobilized rC1-2 and not to GST or to other GST-fusion proteins (Fig 1) from the MC PfEMP1. rF1-2 was tested as an MBP-fusion protein. (B) Immobilized rC1-2 specifically binds CD36 and not other host cell receptors: P-Selectin, E-Selectin (ELAM-1), VCAM-1, and ICAM-1. These receptors do not mediate adherence of MC strain PE. (C) Deletion fragments of rC1-2 [1-233] were amplified by PCR with the appropriate primers, expressed in E coli and immobilized on Ab coated beads directly from 50 μL of bacterial lysate. CD36 binds to the 233 amino acid rC1-2 fragment of MC PfEMP1, denoted rC1-2 [1-233], and to a fragment representing a 41 amino acid deletion from the carboxy terminus, denoted rC1-2 [1-192]. Other fragments of rC1-2, including deletions from the amino or the carboxy end of rC1-2 and mutant forms in which cysteine residues were replaced with serine were also tested. These results are summarized in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3766/4/m_bl_0024f3.jpeg?Expires=1767852667&Signature=tnUyh6lb6St0hw-VvCgNB74vWmyiE2qq8ArYeFp7~tnrMHGbn1mYKtQIVsErCdW3t7hxRuFrcVfkmBtSrNwMBLRI-j7LvImVHhyacLosYT2ESeq8gmCn9FNzZUoOBaPklwhtQZHGImIVXpblPJSrogxr9RAyD67tkKWSnfZQPiZLueO80DdlKU3nY~b-FO~UVomBw1Rc~XrxWp-MoNoD26xcevtJPV0Gf7CCjchu2xPHP33B~D2nNjzW~syrDqO6gdAefenbLUoyXoi1Y8BtUr9HscMXZkU2aJKKtUTqPk3AkLWLVmeY2RGL-o-Fsm5wdqzwU3MKcMeM65RFP5iQRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Blockade and reversal of PE adherence to CD36. Petri dishes coated with CD36 were preincubated with recombinant protein before testing for binding of PE. (A) rC1-2 but not other recombinant proteins, at 100 μg/mL, specifically blocked adherence to CD36 of PE from four phenotypically different P falciparum strains and clones. Strain MC R+ (▪); clone ItG2-ICAM (▨); clone ItG2-G1 (); clone Palo Alto (K−C+) (□). None of the other recombinant proteins affected PE adherence to CD36. Other recombinant proteins of MC PfEMP1 (Figs 1 and 2) did not block adherence of MC strain PE to CD36 (not shown). (B) Concentration-dependent blockade of PE adherence (strain MC) to CD36 with rC1-2 and rC1-2 [1-179]. IC50 values were 1.2 μmol/L for rC1-2 and 0.78 μmol/L for rC1-2 [1-179]. (C) Concentration dependent reversal of MC PE adherence to CD36 with rC1-2. Adherent PE were incubated with GST or rC1-2 [1-233] and remaining adherent cells counted. The IC50 value was 0.5 μmol/L.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3766/4/m_bl_0024f4.jpeg?Expires=1767852667&Signature=bSBpLQvaeNs~qCCsJNDSN8n5N1jsRfzIvW51lfuQ3K1~IpIPVKMaMsGJEHrYCfetE4bFr3PUFPUPHikPMvL79eu3PpT8wi9HbcMer7-NfcWnfPhQrksKWK0spTSzcWjiQJSvyhitZTUany0OeliUIM2sgH64t0VYCOHAjTxi~pRE43t28Cp1IRXdyoyEs2~YhRChvXHJbV0cCVVd~cepqE9aslm9Tau7m7eyf4rQoaSEfsVvm13eIx7Z5xLgXZHd-HGIAendMVratriKsw-kJ9u~pvPU8oGAd2VkD8-5X3Xh8klBKbCSgiNpX4q0Ry90o1eaELMDLgFzWu6d61Pviw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Alignment of the predicted amino acid sequences of the corresponding CD36 binding domain of different var genes. The rC1-2 [1-179] and the rC1-2 [10-151] sequences were amplified by RT-PCR from the expressed var genes of different P falciparum strains and clones (except for the gDNA clone Dd2 var-6). These sequences were detected in gDNA of many strains. Several sequences appeared as expressed var genes (by RT-PCR) in more than one strain. The MC type (first sequence) includes sequences (cDNA, gDNA and RT-PCR) originated from MC parasites (R+, R−, and K−). The ItG/FVO type (second sequence) represents sequences from cDNA of clone FVO, RT-PCR product of ItG-ICAM and gDNA sequences from ItG-ICAM, ItG2-F6, ItG2-G1, FCR3-C5, FCR3-C6, and Palo Alto parasites. The HB3 type (third sequence) includes the RT-PCR products of ItG2-F6 and FCR3-C5 and the gDNA and RT-PCR products of HB3. Other depicted sequences represents expressed var genes (with the exception of Dd2 var-6) amplified with the Uni179 primers from the indicated strains. The emerging consensus sequence of conserved amino acids is depicted on top of the alignment. Highly conserved amino acids are indicated in red, conserved substitutions are indicated by orange, and nonconserved substitutions by green. The conserved cysteine residues are marked by asterisks and additional cysteine residues are shown in blue. These sequence data are available from GenBank under accession numbers AF008978-AF009001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3766/4/m_bl_0024f5.jpeg?Expires=1767852667&Signature=2PPqUjBsc5DTKfvMuX-4u9XWy5OqI~gRajm9RM1zHN-XYbTcJw6nFXs2cWiW6q2FChKFUZTAioZjqKP1YbHSYHLLE18B9Jw7xl~B7OyGAdstmsgq1YLgmrsvOPzCw5tzATDHQML8ZBgoSTSG~inqShDptUryC80P3pm0r9xkf8w25SzeELFCmq32XW2928AKP~EAY~evWPgmcDG9aeYrDePF2gr5GtVFSbqYsCzgaa1comK4DerXve9X2HhwXUQetLlxhsCz~R0K-nKt7XJoJOkTboS8QRT5WNN2oO4prkL11M-DGQvbf~sxkTOxUDD0uwg8lvPvbs54WbfHeq9NTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. Affinity purification of CD36 with immobilized rC1-2 and fragments of rC1-2 [1-233]. Soluble receptors were incubated with bead-immobilized GST fusion recombinant proteins derived from the sequence of MC PfEMP1. Binding of CD36 and other host receptors was detected by Western blotting. (A) CD36 specifically binds to immobilized rC1-2 and not to GST or to other GST-fusion proteins (Fig 1) from the MC PfEMP1. rF1-2 was tested as an MBP-fusion protein. (B) Immobilized rC1-2 specifically binds CD36 and not other host cell receptors: P-Selectin, E-Selectin (ELAM-1), VCAM-1, and ICAM-1. These receptors do not mediate adherence of MC strain PE. (C) Deletion fragments of rC1-2 [1-233] were amplified by PCR with the appropriate primers, expressed in E coli and immobilized on Ab coated beads directly from 50 μL of bacterial lysate. CD36 binds to the 233 amino acid rC1-2 fragment of MC PfEMP1, denoted rC1-2 [1-233], and to a fragment representing a 41 amino acid deletion from the carboxy terminus, denoted rC1-2 [1-192]. Other fragments of rC1-2, including deletions from the amino or the carboxy end of rC1-2 and mutant forms in which cysteine residues were replaced with serine were also tested. These results are summarized in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3766/4/m_bl_0024f3.jpeg?Expires=1767976117&Signature=jLAnuYKJsUK~uBUcvSMT8-jk3dUznQ45I2Zdz64J0WrHfcIBjv8c8LsN42ktkVVdHub4DmazPFz1eanH6dvyJgXhNEO5aNw2Aj6YEMHsuE1nTYjyYu4Pif76s8WZIeVBV1cLIW-YgX5p-tcH5C0XnTCzXU4U5Bzn2DbzJStkL-dosrJzGucwTafjswDSa2DlfS09XOxX4gvtVMA4FDXz1D3cy2G4a54HEwahq-sakJZMfcsGEwAosVE7~6RuuP0WDIDBld8qL6hOIbVDhmoURTOS84fBWZTYKrJJXQzXv6KbZZvGGh1ONz95aXmtrNx~ybe3rVBLdXET7AVmM1YqeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Blockade and reversal of PE adherence to CD36. Petri dishes coated with CD36 were preincubated with recombinant protein before testing for binding of PE. (A) rC1-2 but not other recombinant proteins, at 100 μg/mL, specifically blocked adherence to CD36 of PE from four phenotypically different P falciparum strains and clones. Strain MC R+ (▪); clone ItG2-ICAM (▨); clone ItG2-G1 (); clone Palo Alto (K−C+) (□). None of the other recombinant proteins affected PE adherence to CD36. Other recombinant proteins of MC PfEMP1 (Figs 1 and 2) did not block adherence of MC strain PE to CD36 (not shown). (B) Concentration-dependent blockade of PE adherence (strain MC) to CD36 with rC1-2 and rC1-2 [1-179]. IC50 values were 1.2 μmol/L for rC1-2 and 0.78 μmol/L for rC1-2 [1-179]. (C) Concentration dependent reversal of MC PE adherence to CD36 with rC1-2. Adherent PE were incubated with GST or rC1-2 [1-233] and remaining adherent cells counted. The IC50 value was 0.5 μmol/L.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3766/4/m_bl_0024f4.jpeg?Expires=1767976117&Signature=CmPo~uSxBHlsmIi7JORy84~G~t~fg74Wo2DbnIVbBk-GeoHIShXFGOsd-VosiYsNs2Iy9FmpChWpBuYNF3t8wRQMTHjLGCCnn~lb772NajlrNJR9NXtQf108g8keFugRFdTnKwNSP7xPF3JzNCAjZmrcNaKuxEk3uQApJ5ujTilqSmUTHTL2hqhHa2Esdg95FdblE3EqFmwdcliDG8FEbUTp~iVKJ7xD0n-iF3F4O5wmQlS8QLRET9gRY3bleJRDVOllDfy1v6AoapCpkP4F2qU2mh7q2bxsCPScmGkgQ8v9mbjqRwlcwWbouBRSK6tsrxs4cnBZRUK1BjS4~yCGZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Alignment of the predicted amino acid sequences of the corresponding CD36 binding domain of different var genes. The rC1-2 [1-179] and the rC1-2 [10-151] sequences were amplified by RT-PCR from the expressed var genes of different P falciparum strains and clones (except for the gDNA clone Dd2 var-6). These sequences were detected in gDNA of many strains. Several sequences appeared as expressed var genes (by RT-PCR) in more than one strain. The MC type (first sequence) includes sequences (cDNA, gDNA and RT-PCR) originated from MC parasites (R+, R−, and K−). The ItG/FVO type (second sequence) represents sequences from cDNA of clone FVO, RT-PCR product of ItG-ICAM and gDNA sequences from ItG-ICAM, ItG2-F6, ItG2-G1, FCR3-C5, FCR3-C6, and Palo Alto parasites. The HB3 type (third sequence) includes the RT-PCR products of ItG2-F6 and FCR3-C5 and the gDNA and RT-PCR products of HB3. Other depicted sequences represents expressed var genes (with the exception of Dd2 var-6) amplified with the Uni179 primers from the indicated strains. The emerging consensus sequence of conserved amino acids is depicted on top of the alignment. Highly conserved amino acids are indicated in red, conserved substitutions are indicated by orange, and nonconserved substitutions by green. The conserved cysteine residues are marked by asterisks and additional cysteine residues are shown in blue. These sequence data are available from GenBank under accession numbers AF008978-AF009001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3766/4/m_bl_0024f5.jpeg?Expires=1767976117&Signature=veM00nEYq2EK6zOR69BNXCQoTXYTRqYcYXa~WAPFcG5x93aePsGVvLSXg0seuPUwvsZF10LNc5jq4SVmHyruPdh5FnRGSZWreo9fvJYs8e9mr22buBHUVjo1Ct7od~bllv8phP3SIbgmACIFIknxWxhP29jRtJkQ2ZfBDeKJhMgt3yWMmTW2~ccxiV99GbM9mXSakZu7RMseEbkIsmXMoLiN~gcWmiBOK6L~VUqdBbT6RmGiRBSMJY-suuMIUIdfH4UqD8hi~Awidr1gecBFMhLEH0T-X0g9fr6haXj7uEuHwDawi8PnPtWEJf6ud7pb82w-sAIZHQLUUkM0vWeeSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)