Abstract
Human immunodeficiency virus (HIV)-1 expression in mononuclear phagocytes is associated with multiple functional defects, including phagocytosis. To assess Fcγ receptor (FcγR) function in cells expressing HIV-1, human promonocytic cells (U937) acutely or chronically infected with HIV-1, or stably transfected with a noninfectious reverse transcriptase (RT) defective HIV-1 provirus (Δpol), were treated with phorbol 12-myristate 13-acetate for 48 hours and tested for their ability to ingest sheep erythrocytes coated with IgG (E-IgG). HIV-1–infected or transfected U937 cells ingested 50% to 65% fewer E-IgG than controls despite normal surface expression of FcγRs. HIV-1 specifically impaired FcγR-mediated phagocytosis, as ingestion of complement-coated erythrocytes was unaffected. U937 cells transfected with an env deficient mutant of HIV-1 ingested E-IgG normally, suggesting that the expression of HIV-1 env was required for HIV-1 to inhibit FcγR-mediated phagocytosis. Expression of HIV-1 in U937 cells was associated with an increased accumulation of intracellular cyclic adenosine monophosphate (cAMP); addition of the adenylate cyclase inhibitor 2′,5′-dideoxyadenosine to these cells decreased intracellular cAMP levels to that of controls and restored FcγR-mediated phagocytosis. Addition of either interferon (IFN)-γ or an inhibitor of cAMP-dependent protein kinase A (KT 5720) to HIV-1–transfected U937 cells also restored FcγR-mediated phagocytosis. Expression of HIV-1 induces a specific defect of FcγR function in mononuclear phagocytes that correlates with increased levels of cAMP, and can be corrected by pharmacologic manipulation.
INFECTION WITH human immunodeficiency virus (HIV)-1 leads to a profound and progressive state of immune deficiency, particularly in the number and function of CD4+ lymphocytes.1 Mononuclear phagocytes are also targets of HIV-12 and may serve as reservoirs for HIV-1.3-5 While the viral burden of circulating blood mononuclear cells is low (0.0025% to 2.5% of cells are infected) consisting mostly of CD4+ lymphocytes,6,7 the percentage of tissue macrophages infected with HIV-1 is appreciable, particularly in the brain (1% to 10%),8,9 lymph nodes (10%),10 and the lung (10% to 50%).11 This raises the question of whether expression of HIV-1 has direct deleterious effects on macrophage function.
Receptors for the Fc portion of IgG (Fcγ receptors; Fcγ Rs) mediate phagocytosis of IgG-opsonized particles and clearance of immune complexes. In HIV-1–infected patients, clearance of IgG-coated erythrocytes is impaired,12 suggesting a decline in FcγR function in patients with acquired immune deficiency syndrome (AIDS). Furthermore, mononuclear phagocytes from HIV-1–infected patients have decreased FcγR function in vitro.13-15 This decline in receptor function may have important consequences for host defense and susceptibility to infections, and may reflect direct or indirect effects of HIV-1 infection. It is not clear whether decreased FcγR function reflects decreased surface expression of FcγRs, as suggested by a study using monocyte-derived macrophages infected with HIV-1 in vitro16 or abnormally functioning surface FcγRs, because FcγR expression on monocytes isolated from HIV-infected individuals is reportedly normal.14 17
The aim of this study was to assess the direct effect of HIV-1 expression on FcγR-mediated phagocytosis. To avoid working with heterogeneous cell populations (ie, consisting of HIV-infected and uninfected cells), we studied U937 cells (a human mononuclear phagocyte cell line), which were latently infected with HIV-1 or expressed HIV-1 gene products. Our results indicate that infection with HIV-1 leads to impairment of FcγR-mediated phagocytosis, and that this impairment is mediated by accumulation of cyclic adenosine monophosphate (cAMP).
MATERIALS AND METHODS
Cells. U937 cells were obtained from the American Type Tissue Collection (Rockville, MD) and U1 cells were obtained from the AIDS Research and Reference Reagent Program (Bethesda, MD) and are described elsewhere.18,19 UΔpol cells were generated by electroporation of 107 U937 cells with 5 mg of pHXBΔpol, a noninfectious proviral plasmid.20 A deletion in the pol coding region abrogates reverse transcriptase (RT) activity of UΔpol cells; consequently, cells transfected pHXBΔpol express HIV-1 proteins, but do not produce infectious virions. UΔenv cells were generated by transfection of U937 cells with an env deficient mutant of Δpol. 21 U937/LAV cells are U937 cells that survived acute infection with HIV-1 (strain LAV) and produce high constitutive levels of HIV-1 as determined by RT assay.
Phagocytosis assays. A total of 2.5 × 105 U937 cells were incubated in 12-well dishes at 37°C for 48 hours in RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Intergen, Purchase, NY) and 40 nmol/L phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO) to induce differentiation to a phagocytic phenotype.22 Interferon (IFN)-γ (Collaborative Biomedical Products, Bedford, MA), 2′,5′-dideoxyadenosine (ddAdo; Pharmacia, Piscataway, NJ), or KT 5720 (Calbiochem, La Jolla, CA) were added at the indicated concentrations. After 48 hours, sheep erythrocytes opsonized with IgG23 or C3bi24 were added for 1 hour at 37°C, the cells were washed, fixed in 3.7% formaldehyde, and stained with acridine orange (10 μg/mL) for 20 minutes to distinguish bound from ingested opsonized erythrocytes.23 The phagocytic index (PI) was determined by counting the number of ingested erythrocytes per 100 macrophages, as described.23 Percent phagocytosing cells denotes the number of cells that ingested ≥1 target. Some results were expressed in percent of the PI of uninfected U937 controls.
cAMP measurements. A total of 5 × 106 adherent U937, U1, UΔpol, or UΔenv cells incubated with 40 nmol/L PMA as described above were used for determination of cAMP content. Measurements of intracellular cAMP were performed using a commercially available assay (Amersham, Arlington Heights, IL) according to manufacturer's recommendations.
Cell surface FcγR expression. A total of 106 U937, U1, or UΔpol cells were incubated with 40 nmol/L PMA as described and stained with the following fluorescein isothiocyanate (FITC)-labeled antibodies, or isotype-matched controls, for 20 minutes at 4°C: monoclonal antibody (MoAb) 32.2 (anti-Fcγ RI), MoAb IV.3 (anti-FcγRII), or MoAb 3G8 (anti-Fcγ RIII), all from Medarex (Annandale, NJ). Flow cytometry analyses were performed on a Coulter Epics 753 (Coulter Corp, Miami, FL).
RESULTS
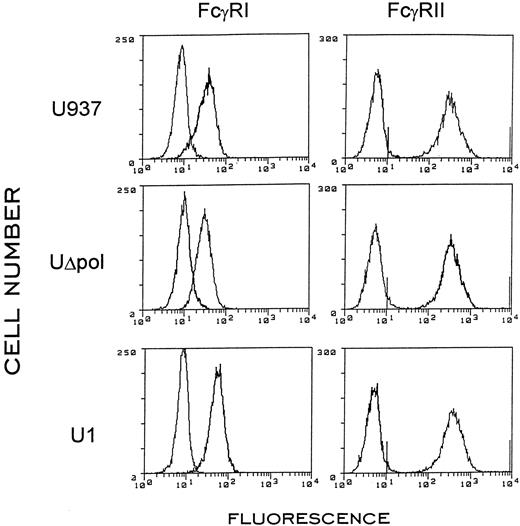
Expression of HIV-1 in U937 cells decreases phagocytosis of E-IgG, but not of E-C3bi. We measured the ability of U937 cells, which were acutely (U937/LAV) or chronically (U1) infected with HIV-1 or transfected with a noninfectious HIV-1 provirus (UΔpol), to ingest E-IgG. To render U937 cells capable of phagocytosis, we incubated them with 40 nmol/L PMA for 48 hours before each experiment.22 The phagocytic indices of U937/LAV and U1 cells and three different UΔpol cell clones were decreased by 51% to 66% compared with U937 control cells (Table 1). Similarly, the ability of HIV-1–infected or transfected U937 cells to ingest 1 or more E-IgG was decreased by 27% to 64% compared with U937 control cells. The impairment of phagocytosis was not due to decreased surface expression of Fcγ receptors, as flow cytometry showed equivalent levels of surface expression of FcγRI and II (Fig 1). Consistent with earlier findings,25 we could not detect surface expression of FcγR III in U937 cells (not shown).
Surface expression of FcγR I and II in U937, UΔpol, and U1 cells. Flow cytometry of the indicated cells incubated with anti-FcγR IgG was performed after a 48-hour incubation with 40 nmol/L PMA. The left curves represent cells incubated with FITC-labeled isotype matched control antibodies.
Surface expression of FcγR I and II in U937, UΔpol, and U1 cells. Flow cytometry of the indicated cells incubated with anti-FcγR IgG was performed after a 48-hour incubation with 40 nmol/L PMA. The left curves represent cells incubated with FITC-labeled isotype matched control antibodies.
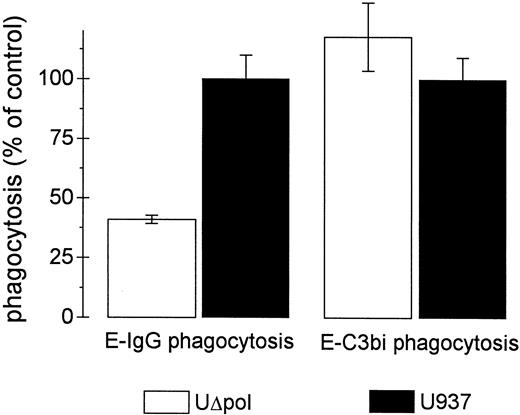
To test whether the phagocytic impairment was specific for Fcγ receptors, erythrocytes opsonized with C3bi (E-C3bi) were used as alternative phagocytic targets. UΔpol cells ingested equal numbers of E-C3bi compared with U937 control cells (Fig 2). In a separate experiment, phagocytosis of E-C3bi by U937 or UΔpol cells was inhibited by >90% in the presence of EDTA, consistent with earlier findings that ingestion of E-C3bi was mediated primarily by complement receptor-3 (Mac-1/αMβ2 ) and requires the presence of divalent cations.26
Expression of HIV-1 does not affect phagocytosis of E-C3bi. Phagocytosis assays were performed as described in Materials and Methods. The mean phagocytic indices of untransfected U937 control cells for E-IgG and E-C3bi were 237 ± 21 and 176 ± 15, respectively. Data represent the mean ± standard error of mean (SEM) (n = 3).
Expression of HIV-1 does not affect phagocytosis of E-C3bi. Phagocytosis assays were performed as described in Materials and Methods. The mean phagocytic indices of untransfected U937 control cells for E-IgG and E-C3bi were 237 ± 21 and 176 ± 15, respectively. Data represent the mean ± standard error of mean (SEM) (n = 3).
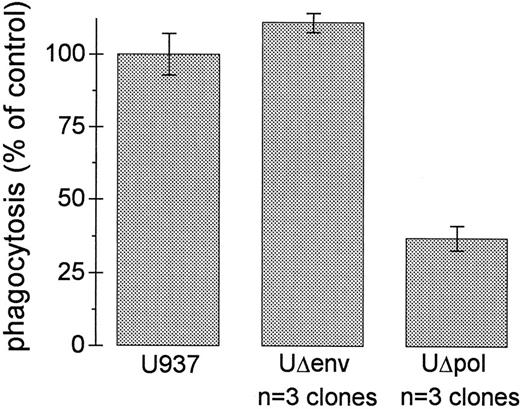
HIV-1 Env decreases the phagocytic capacity of U937 cells. The envelope glycoprotein of HIV-1 (gp120) has been reported to impair various cellular functions.15,27-30 To test whether expression of HIV-1 env in U937 cells affects FcγR function, we assessed E-IgG phagocytosis in U937 cells transfected with a Δpol construct that is env deficient (Δpol-Δenv).21 U937Δpol-Δenv cells showed levels of phagocytosis of E-IgG that equaled or exceeded that of U937 control cells (Fig 3). Thus, one or more products of the env gene is required for the impairment in Fcγ receptor-mediated phagocytosis by HIV-1.
Impairment of FcγR-mediated phagocytosis requires HIV-1 env. Ingestion of E-IgG was performed as described in Materials and Methods using U937 cells and three different UΔpol and UΔenv clones. Data represent the mean ± SEM (n = 6).
Impairment of FcγR-mediated phagocytosis requires HIV-1 env. Ingestion of E-IgG was performed as described in Materials and Methods using U937 cells and three different UΔpol and UΔenv clones. Data represent the mean ± SEM (n = 6).
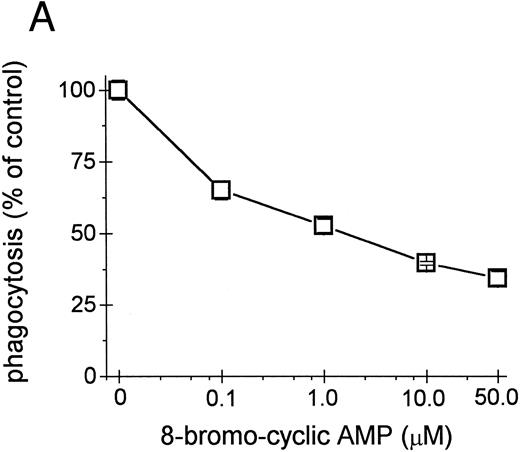
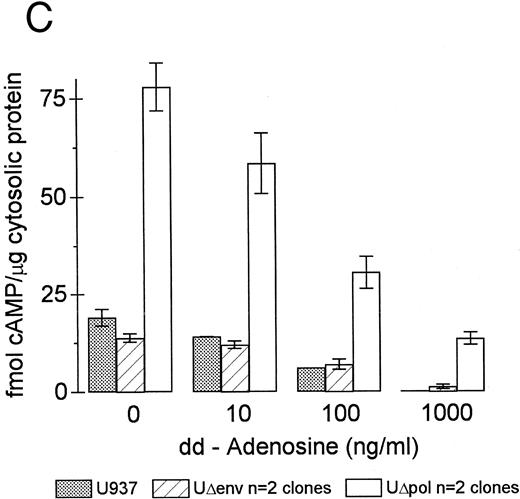
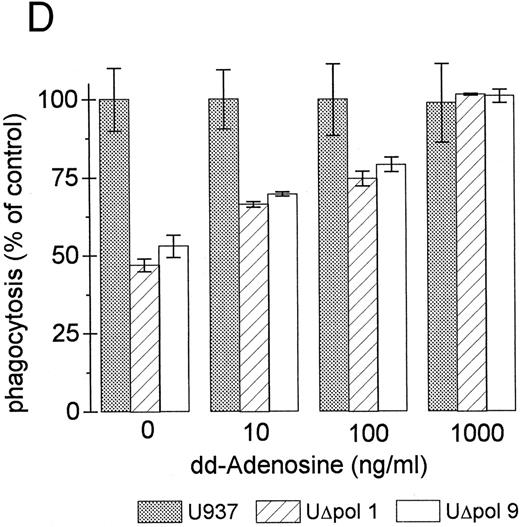
Inhibition of intracellular cAMP formation or cAMP-dependent protein kinase restores the phagocytic capacity of UΔpol cells. Addition of a cell-permeant analog of cAMP decreased FcγR-mediated phagocytosis in U937 cells (Fig 4A), consistent with other reports.31,32 We reasoned that expression of HIV-1 env might decrease FcγR-mediated phagocytosis through a cAMP-dependent mechanism. Intracellular cAMP levels were four times higher in UΔpol cells and 12 times higher in U1 cells compared with nontransfected U937 cells or Δpol-Δenv transfected U937 cells (Fig 4B), similar to results of a previous study33 using a T-lymphoblast cell line. Addition of the adenylate cyclase inhibitor ddAdo decreased intracellular cAMP content (Fig 4C) of UΔpol cells and restored FcγR-mediated phagocytosis of these cells to control levels (Fig 4D).
cAMP inhibits Fcγ R-mediated phagocytosis in U937 cells. (A) Phagocytosis of E-IgG by U937 cells was performed after preincubation for 48 hours in the presence of the indicated concentrations of 8-bromoadenosine-3′:5′-cyclic-monophosphate and 40 nmol/L PMA. (B) cAMP levels in HIV-1–expressing U937 cells and controls. (C) cAMP levels in the indicated cell lines incubated for 48 hours in the absence or presence of the adenylate cyclase inhibitor, 2′-5′-dideoxyadenosine. (D) Phagocytosis of E-IgG by either U937 or UΔpol cells incubated for 48 hours in the absence or presence of the adenylate cyclase inhibitor, 2′-5′-dideoxyadenosine. Data represent the mean ± SEM of three to six experiments.
cAMP inhibits Fcγ R-mediated phagocytosis in U937 cells. (A) Phagocytosis of E-IgG by U937 cells was performed after preincubation for 48 hours in the presence of the indicated concentrations of 8-bromoadenosine-3′:5′-cyclic-monophosphate and 40 nmol/L PMA. (B) cAMP levels in HIV-1–expressing U937 cells and controls. (C) cAMP levels in the indicated cell lines incubated for 48 hours in the absence or presence of the adenylate cyclase inhibitor, 2′-5′-dideoxyadenosine. (D) Phagocytosis of E-IgG by either U937 or UΔpol cells incubated for 48 hours in the absence or presence of the adenylate cyclase inhibitor, 2′-5′-dideoxyadenosine. Data represent the mean ± SEM of three to six experiments.
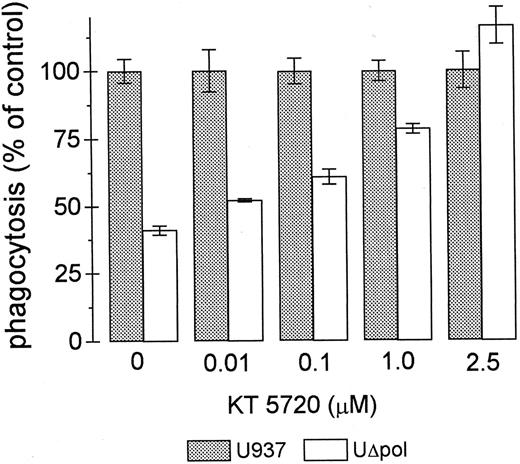
Many of the intracellular actions of cAMP are mediated by cAMP-dependent protein kinase (PKA). We tested whether the decrease in FcγR-mediated phagocytosis in UΔpol cells was reversed by inhibition of PKA activity. Addition of the PKA inhibitor KT 572034 to UΔpol cells restored FcγR-mediated phagocytosis to control levels (Fig 5), but did not significantly affect the phagocytic capacity of nontransfected U937 cells (not shown).
The PKA inhibitor KT5720 restores phagocytosis of E-IgG by UΔpol cells. Phagocytosis assays were performed with the indicated concentrations of the PKA inhibitor KT5720 as described in Materials and Methods. Data represent the mean ± SEM (n = 3).
The PKA inhibitor KT5720 restores phagocytosis of E-IgG by UΔpol cells. Phagocytosis assays were performed with the indicated concentrations of the PKA inhibitor KT5720 as described in Materials and Methods. Data represent the mean ± SEM (n = 3).
IFN-γ restores the phagocytic capacity of UΔpol cells. IFN-γ has been shown to increase human monocyte phosphodiesterase activity35 and to downregulate membrane adenylate cyclase activity,36 both of which might decrease intracellular cAMP concentrations. We incubated PMA-treated UΔpol cells with or without IFN-γ for 48 hours and quantitated phagocytosis of E-IgG. Addition of IFN-γ decreased intracellular cAMP concentrations and increased phagocytosis of E-IgG by UΔpol cells (Fig 6), and U1 cells (not shown), whereas it had no significant effect on phagocytosis of E-IgG by nontransfected U937 cells. Flow cytometry showed that IFN-γ treatment did not increase expression of FcγRIs and FcγRIIs in PMA-treated UΔpol and U1 cells (not shown).
Restoration of FcγR-mediated phagocytosis by IFN-γ. Phagocytosis of E-IgG by UΔpol cells and intracellular cAMP concentrations were measured as described in Materials and Methods after incubation for 48 hours with the indicated concentrations of recombinant IFN-γ. Data represent the mean ± SEM (n = 3).
Restoration of FcγR-mediated phagocytosis by IFN-γ. Phagocytosis of E-IgG by UΔpol cells and intracellular cAMP concentrations were measured as described in Materials and Methods after incubation for 48 hours with the indicated concentrations of recombinant IFN-γ. Data represent the mean ± SEM (n = 3).
DISCUSSION
HIV-1 infection is associated with multiple functional defects in mononuclear phagocytes,2 such as decreases in chemotactic activity,27,37-39 clearance of IgG-labeled targets,12 antigen presentation,40 and intracellular killing of Candida spp.,41,42,Toxoplasma gondii,43,44 and Aspergillus fumigatus.45 Diminished phagocytosis of a variety of particulate targets by mononuclear phagocytes from HIV-1–positive individuals has been reported in some,12-14 but not all studies.39,46,47 Some of these differences may reflect heterogeneity in the cell population studied, possibly as a result of using cells from patients at different stages of HIV-1 infection and/or with different viral burdens. For example, production of viral proteins p24 and gp120 was undetectable in alveolar macrophages from 15 HIV-1–positive patients.48 When cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF ) and tumor necrosis factor (TNF )-α, 30% to 60% of cells from these patients produced viral proteins and 60% to 80% expressed viral RNA,48 suggesting that the majority of theses cells are latently infected. In addition, viral burden increases with progression from the asymptomatic carrier state to AIDS.1,49 50 Similarly, heterogeneity in the population of infected cells also occurs in primary cells infected with HIV-1 in vitro, as virus expression varies from 28%16 to 75%51 of cells after infection with HIV-1 in vitro. Therefore, the use of a uniform population of HIV-1–expressing mononuclear phagocytes, as described in this study, tests the direct effect of HIV-1 on phagocytic function. These cells may serve as a model system to study the effects of HIV-1 on tissue macrophages, particularly during advanced stages of the disease.
Little is known about the mechanisms by which HIV-1 causes alterations of mononuclear phagocyte function. In the present study, we show that expression of HIV-1 env impairs Fcγ receptor-mediated phagocytosis, which is correlated with an enhanced accumulation of cAMP, an effect which is reversed by agents that inhibit cAMP production or that block its effects. These findings are consistent with reported depressant effects of cAMP on phagocytosis by an unknown mechanism.31,32 Precisely how expression of HIV-1 leads to increased accumulation of cAMP is not known, but our data suggest an involvement of HIV-1 env (Fig 4B). Perhaps binding of gp120 to CD4 or its coreceptor, CCR-5, a member of the chemokine receptor family, activates adenylate cyclase by stimulating a G protein. Interestingly, addition of macrophage inflammatory protein 1 alpha (MIP-1α), a known ligand of CCR-5, to the human M07e cell line enhances the cAMP content of these cells52 and exposure to picomolar concentrations of gp120 increases the cAMP content of microglial cells.53 We envision that expression of HIV-1 env leads to decreased phosphodiesterase activity and/or upregulation of adenylate cyclase activity, both of which can be reversed by IFN-γ.35 36 This may explain our finding that IFN-γ restores FcγR-mediated phagocytosis in HIV-1–expressing cells. Elucidating the cellular changes that accompany HIV-1 infection of mononuclear phagocytes may lead to the development of new therapeutic strategies for the treatment of the immune defects that are characteristic of HIV-1 infection.
Supported by an Investigatorship from Arthur N. Saydman Trust Fund, New York, NY for Research in Septicemia in honor of Dr Harold C. Neu at Columbia University (to C.A.T.); a Research Fellowship from the Lucille P. Markey Charitable Trust, Miami, FL (to C.A.T.); Grant No. HL54164 (to S.G.), Grant No. AI20516 (to S.C.S), and Grant No. HL43528 (to D.K.W.) from the National Institutes of Health, Bethesda, MD; Grant No. FI4P-CT95-0029 from the European Communities, Brussels, Belgium (to B.L.Z); and Grant No. E/B41G/T0347/T5920 from the German Ministry of Defense, Bonn, Germany (to B.L.Z). S.G. is an Established Investigator of the American Heart Association.
Address reprint requests to Christian A. Thomas, MD, Division of Medical Oncology, Columbia Presbyterian Medical Center, MHB-6-435, 177 Ft Washington Ave, New York, NY 10032.