Abstract
Fourteen patients with cytogenetic relapse of chronic myeloid leukemia (CML) after transplantation with unmanipulated bone marrow were treated with α-2a–interferon. There were eight men and six women, median age, 33 years. Twelve patients received marrow from a related allogeneic donor and two received marrow from a syngeneic donor. The median percentage of Ph-positive metaphases at the time of starting interferon was 55% (10% to 87%). Daily interferon was started at a dose of 1 to 3 × 106 U/M2/d, depending on initial blood counts and was adjusted as tolerated to maintain the white blood count in the range of 2,000 to 3,000/μL and the platelet count greater than 60,000/μL. After a stable cytogenetic remission was achieved, the interferon dose was decreased to a maintenance level. Twelve patients achieved a complete cytogenetic remission on at least one occasion. Median time to achieve a complete cytogenetic remission was 7.5 months (range, 1.5 to 12). Eight patients remain in cytogenetic remission for 10+ to 54+ months from the time of first documented remission. After complete cytogenetic remission was established, nine patients were tested for the presence of the mRNA transcript of the bcr/abl fusion gene by polymerase chain reaction (PCR) testing. Four patients were PCR-negative on at least one occasion: two patients were PCR-negative on a single occasion; one patient had serial tests, which were PCR-negative; and one patient had serial PCR-negative peripheral blood tests with a single PCR-positive bone marrow obtained concurrently with a negative peripheral blood test. Median follow-up time for all patients is 44 months (range, 20 to 64). Interferon was generally well tolerated; only one responding patient was unable to continue interferon because of toxicity. Interferon induces durable cytogenetic remissions in a significant proportion (57%) of patients with cytogenetic relapse following bone marrow transplantation (BMT) without causing life-threatening toxicities.
THE REAPPEARANCE of the Philadelphia (Ph) chromosome in unstimulated bone marrow metaphases after bone marrow transplantation (BMT) for chronic myeloid leukemia (CML) may predate early relapse or may be a transient phenomenon, depending on timing from transplant.1 Early after BMT, the Ph chromosome may be present in low frequency without apparent clinical consequence. Emergence of the Ph chromosome 6 or more months after transplant, however, generally heralds the onset of clinical relapse within the next 2 years.2
α-Interferon therapy of hematologic relapse after BMT is generally well tolerated and produces durable complete cytogenetic responses in approximately 25% of patients.3 In the case of cytogenetic relapse, defined as recurrence of the Ph clone without blood count or bone marrow morphology abnormalities, the tumor burden is lower and a greater proportion of donor cells is available to participate in the graft versus leukemia response. Under these circumstances, interferon may be more effective than in hematologic relapse. To test this hypothesis, a phase II study of interferon was undertaken in patients with cytogenetic relapse of CML after BMT.
MATERIALS AND METHODS
Patients.To be eligible for study entry, all patients had to have cytogenetic relapse of CML after BMT. Criteria for cytogenetic relapse included: (1) normal peripheral smear and bone marrow morphology, (2) presence of the Ph chromosome in unstimulated bone marrow cytogenetic studies at least 6 months from BMT or, if less than 6 months from transplant, a rising proportion (>50%) of Ph positive metaphases, and (3) persistence of the Ph clone after discontinuation of immunosuppression for at least 1 month. Patients could not have active graft-versus-host disease (GVHD) requiring treatment with both cyclosporine and prednisone or azathioprine alone or in combination, platelet count <80,000/μL, absolute neutrophil count <1,000/μL, severe depression, significant cardiac disease, or Karnofsky score <80%. Most patients lived outside Seattle and therefore were managed by telephone in conjunction with the primary hematologist/oncologist. All patients were entered and treated according to a protocol approved by the Institutional Review Board and gave written informed consent.
Patient characteristics.From the time the study was initiated through the date the last patient was entered, 28 patients transplanted at Fred Hutchinson Cancer Research Center were identified with cytogenetic relapse. Ten patients were not registered on protocol because they did not meet the eligibility requirements. Of 18 patients registered on study, 14 patients with cytogenetic relapse of CML were eligible to receive interferon and are evaluable for response and toxicity (Table 1) and four patients were deemed ineligible. There were eight men and six women, median age, 33 years (range, 12 to 60). All patients were in chronic phase at the time of BMT. Twelve patients received marrow from a related donor (11 matched and 1 one-antigen mismatched) and 2 patients received syngeneic marrow. Six patients received marrow from a donor of the opposite sex.
The median time from diagnosis to BMT was 7.5 months (range, 1 to 119). Twelve patients received cyclophosphamide (120 mg/kg) and fractionated total body irradiation1 and two patients received busulfan and cyclophosphamide for pretransplant conditioning.4 All recipients of allogeneic marrow were treated with cyclosporine and short methotrexate for prophylaxis of GVHD.5 None of the patients received T-cell–depleted marrow.
Three patients received interferon before marrow transplantation. Patient 4 received α interferon for 8 months before transplantation and achieved a hematologic, but not a cytogenetic response. Six weeks before transplantation, patient 9 was treated with a combination of α- and γ-interferon without hematologic or cytogenetic response. Patient 15 received α-interferon for 7 years and had good hematologic control, but no cytogenetic response; marrow transplantation was performed when hematologic control was lost.
The Philadelphia chromosome was detected a median of 15.5 months (1 to 57) after BMT, and interferon was started a median of 3.5 months (range, 0.5 to 30) after the first evidence of cytogenetic relapse. Ten patients did not start interferon until the cytogenetic analysis showed greater than 50% Ph-positive metaphases, and four patients started interferon with less than 50% Ph-positive metaphases. The median percentage of Ph-positive metaphases at the time interferon was started was 55% with a range of 10% to 87%.
Four patients (patients 11, 13, 14, and 17) were still on cyclosporine at the time cytogenetic relapse was first detected. In all four patients, cyclosporine was discontinued and follow-up cytogenetic analyses showed persistent or increasing percentage of Ph-positive metaphases. Patient 11 did not respond to interferon and progressed to blast crisis. Patients 13, 14, and 17 were off cyclosporine for 2, 3, and 18 months, respectively before starting interferon.
Treatment schema.Induction: α-2a-interferon (Roferon) in the lyophilized form was supplied by Hoffmann-LaRoche (Nutley, NJ). Patients were taught to reconstitute the drug and to self-administer a subcutaneous injection. The median starting dose of interferon was 3 × 106 U/M2/d (range, 0.5 to 3). Blood counts were checked weekly and the dose was adjusted as tolerated to produce a white blood cell count (WBC) in the range of 2,000 to 3,000/μL and platelet count >60,000/μL. Bone marrow for cytogenetic studies was requested every 3 months during induction. If a complete cytogenetic response was documented on two consecutive occasions, a maintenance schedule was started and bone marrow and/or peripheral blood was tested for presence of the bcr/abl rearrangement by polymerase chain reaction (PCR).
Maintenance.Usually the initial maintenance dose of interferon consisted of the dose of interferon resulting in a complete cytogenetic response administered 5 days per week instead of daily. If a patient had problems with toxicity on the maintenance schedule, the dose of interferon was reduced by approximately 25%.
After documenting that the initial maintenance dose would maintain the cytogenetic remission, an attempt was made to minimize the dose and frequency of interferon administration. If the 5-day per week interferon schedule maintained a complete cytogenetic response, the schedule was changed to every other day. Ultimately, the smallest possible dose that would maintain a remission was administered.
Dose modifications.Interferon was temporarily held for any grade 3 or 4 nonhematologic toxicity or if the absolute neutrophil count was <800/μL or the platelet count was <60,000/μL. After recovery of blood counts or improvement of nonhematologic toxicity was documented, interferon was restarted at 50% of the previous dose.
Cytogenetic analyses.Bone marrow examinations were performed every 3 months during induction and every 3 to 4 months during the first 12 to 18 months of maintenance therapy. Thereafter, marrows were requested every 6 to 12 months. Fresh heparinized marrow and peripheral blood were generally sent to Seattle by overnight shipment. Cytogenetic analyses were performed as previously described.3 All analyzable metaphase cells were scored until a maximum of 50 was reached. Because the mitotic index was often markedly reduced after patients started interferon, only 20 metaphases were scored in most cases.
Y body analysis.In selected patients, the Y body was assayed by in situ hybridization according to previously reported methods.6
Reverse transcription (RT)-PCR amplification.After the first complete cytogenetic remission was documented, peripheral blood or bone marrow samples were tested for chimeric bcr/abl mRNA by RT-PCR. Thereafter, RT-PCR testing was obtained concurrently with cytogenetic testing whenever possible, usually every 3 to 6 months. RNA preparation and the RT-PCR assay for bcr/abl were performed as previously described.7
Response criteria.Complete cytogenetic response is defined as complete disappearance of the Philadelphia chromosome on at least one test. Because all patients started with a normal hemogram, “hematological response” was not considered relevant.
RESULTS
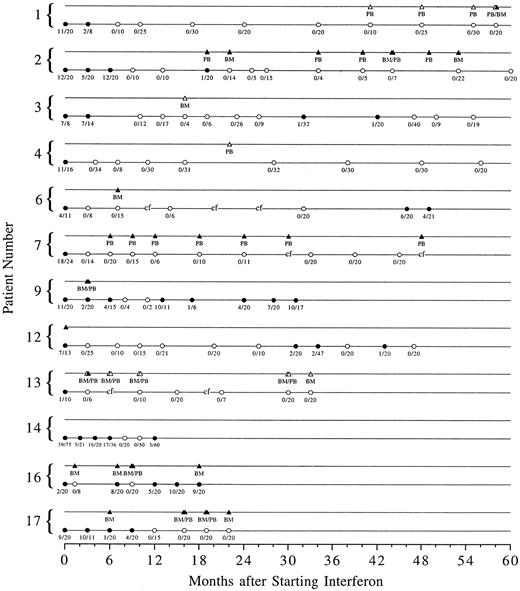
Cytogenetic response.Complete cytogenetic remission was documented on at least one marrow sample in 12 of 14 patients (Fig 1). Of the 12 patients who achieved a complete cytogenetic remission, two patients are no longer on interferon. One patient (patient 9) stopped interferon because of toxicity and one (patient 14) chose to go off study to receive treatment with donor lymphocyte infusions when a small percentage of Ph-positive metaphases was detected after two Ph-negative studies. Of the 10 patients who remain on interferon, six (patients 1, 2, 4, 7, 13, and 17) remain in continuous complete cytogenetic remission for 10+ to 54+ months since the first remission bone marrow or 22 months to 5 years after starting interferon. Two patients (patients 3 and 12) had transient reemergence of the Ph clone, which disappeared after the interferon dose was increased. Two patients (patients 6 and 16) had a cytogenetic relapse and have not yet reestablished a complete remission. Patient 6 stopped interferon on his own; 4 months after interferon was restarted, the percentage of Ph-positive metaphases has decreased from 30% to 19%. Interferon was discontinued in patient 16 because a skin rash, ultimately found to be due to scabies, was considered to be caused by GVHD. He continues on interferon without a significant change in the percentage of Ph-positive metaphases.
Serial cytogenetic and PCR data for the 12 patients who achieved a complete cytogenetic remission on at least one occasion. Solid circles represent Ph-positive metaphases in the bone marrow and open circles represent Ph-negative marrow cytogenetics. The number of Ph-positive metaphases over the total number of metaphases counted is shown below each circle. Corresponding solid or open triangles represent PCR positive or negative testing of peripheral blood (PB) or bone marrow (BM). “cf” signifies bone marrow culture failure.
Serial cytogenetic and PCR data for the 12 patients who achieved a complete cytogenetic remission on at least one occasion. Solid circles represent Ph-positive metaphases in the bone marrow and open circles represent Ph-negative marrow cytogenetics. The number of Ph-positive metaphases over the total number of metaphases counted is shown below each circle. Corresponding solid or open triangles represent PCR positive or negative testing of peripheral blood (PB) or bone marrow (BM). “cf” signifies bone marrow culture failure.
The median time to achieve a complete cytogenetic remission was 7.5 months (range, 1.5 to 12) and the median duration of response has not been reached with a median follow-up of 44 months (range, 20 to 64).
The Ph-negative population is of donor origin in all four patients (patients 2, 7, 9, and 12) who had a donor of the opposite sex and who achieved a complete cytogenetic remission.
Two patients had no response to interferon. Patient 11, the youngest in the series, relapsed early (day 36) and progressed to blast crisis and died within 19 months of BMT. Patient 15 had received interferon for 7 years and had hematologic control, but no cytogenetic response before BMT. Posttransplant, interferon appeared to stabilize the progression of the Ph-clone for approximately 8 months, but eventually the percentage of Ph-positive metaphases increased to 90% and the patient was referred for donor lymphocyte infusion while still in cytogenetic relapse. She is now in a complete cytogenetic and molecular remission, but suffers from mucosal GVHD and requires immunosuppression with cyclosporine and prednisone.
RT-PCR response.Nine of the 12 patients who achieved a complete cytogenetic remission were evaluated for presence of bcr/abl mRNA as detected by RT-PCR (Fig 1). Two patients (patients 3 and 4) had a negative test on one occasion. One patient (patient 1) has been consistently negative in peripheral blood over 17 months, but had one bone marrow sample tested, which was positive at the same time the peripheral blood was negative. One patient (patient 13) has been consistently negative in peripheral blood and bone marrow over 27 months. Of the five patients who have positive PCR testing, two (patients 6 and 16) were tested only once, and three have had serial positive tests.
Survival.One (patient 11) of the two nonresponders died in blast crisis as described above. The other patient (patient 15) was salvaged with donor lymphocyte infusions and has both a complete cytogenetic and molecular response; this patient also had received interferon pretransplant for 7 years. The remaining 12 patients who had a complete response on at least one occasion remain alive. There were no treatment-related deaths.
Induction dose.The median tolerated average daily induction dose was 2.95 × 106 U/M2/d with a range of 0.45 to 4.8 × 106 U/M2/d.
Toxicity.Table 2 summarizes the nonhematologic toxicities. Most patients experienced a transient flu-like syndrome consisting of fever, myalgias, headache, nausea, and somnolence. These effects generally resolved approximately 2 to 4 weeks after starting interferon. Most patients noted a chronic low-grade fatigue with long-term therapy, but were able to maintain pretreatment jobs and other activities. There were no episodes of grade 4 toxicity.
Only one patient in this series could not tolerate an adequate dose of interferon to maintain a remission. Patient 9 had to stop interferon because of grade 3 weight loss and fatigue. Although interferon induced a complete cytogenetic remission, the Ph population recurred when the interferon was held. After interferon was restarted, the percent of Ph-positive metaphases declined, but recurrent toxicity required dose reduction with a subsequent increase in the percentage of Ph-positive metaphases. This patient ultimately received donor lymphocyte infusion (DLI) and had GVHD of the skin, but achieved a complete cytogenetic remission.
DISCUSSION
The reappearance of the Philadelphia chromosome after BMT for CML is worrisome for both patient and clinician. Although the Philadelphia chromosome may emerge transiently in the first 3 months after transplantation, its presence at that time in the absence of other findings does not necessarily herald relapse. Likewise, when patients are still immunosuppressed with cyclosporine, the Philadelphia chromosome may reappear, only to disappear after immunosuppression is discontinued. This phenomenon was observed in four of 14 other patients with cytogenetic relapse who were not eligible for this study.
When a spontaneous cytogenetic remission does not occur after cyclosporine is discontinued, other therapeutic interventions are usually entertained. We and others have previously reported that interferon therapy is well tolerated and results in complete cytogenetic remissions in hematologic relapse of chronic phase CML after BMT.3 8-10 In our experience with interferon in that setting, a complete cytogenetic remission was achieved and maintained in 29% of patients or twice that reported for patients treated with interferon for de novo CML. Based on these results, we hypothesized that interferon may be more effective in cytogenetic relapse where the tumor burden is smaller and there is a greater proportion of donor T cells than in hematologic relapse. Under these conditions, it is more likely that a biologic response modifier such as interferon would be successful in suppressing the malignant clone, not only by direct inhibition, but also by induction of the graft versus leukemia effect through immune modulation of the donor population.
In this small, but prospective, series of patients with cytogenetic relapse, a substantial proportion of patients (80%) treated with interferon achieved a complete cytogenetic remission on at least one occasion and eight of 10 patients who continue on interferon, or 57% of all treated patients, remain in remission for up to 5 years after starting interferon. This twofold difference in durable complete cytogenetic response rates between cytogenetic and hematologic relapse supports the theory that disease burden is important. However, the fact that most patients do not achieve a molecular remission and require maintenance interferon to sustain a complete response suggests that interferon alone is not adequate to eradicate the malignant clone even in the setting of cytogenetic relapse. Whether persistence of the molecular marker in patients treated with interferon has an impact on survival remains unclear.
Another therapeutic option for cytogenetic relapse is infusion of donor lymphocytes (DLI). Multiple reports11-22 have demonstrated the efficacy of DLI alone or in combination with interferon, in eradicating the Ph-positive clone after relapse of CML after BMT. In addition to a high complete cytogenetic response rate, more than half of the complete responders also achieve a molecular remission by PCR testing. Unfortunately, DLI may result in GVHD and marrow aplasia in a significant percentage of cases. Deaths related to these complications may occur in up to 20% of patients within the first year after treatment. Long-term data for duration of response, survival, and toxicities is not yet available from most published studies.
Disease burden also appears to be a significant factor in the outcome of DLI. Patients with minimal residual disease, in either cytogenetic or PCR relapse, can receive lower doses of donor lymphocytes and achieve complete cytogenetic and molecular remissions without developing life-threatening GVHD or aplasia.22 Interestingly, some patients who achieved a cytogenetic, but not a molecular remission, were treated with short course interferon, which induced a molecular remission.
Our current treatment algorithm for relapse of CML is based on the extent of tumor burden. Patients who have only PCR evidence of disease during the first 6 to 12 months after BMT have a risk for relapse of 40% to 60% within the first 2 years after BMT.7 These patients are offered 1 year of treatment with interferon. Eligible patients with cytogenetic-only relapse are treated with interferon as described in this study. If a complete cytogenetic response to interferon is not achieved after 12 months of treatment or if there is evidence of significant cytogenetic progression, patients are referred for DLI. Patients with hematologic relapse currently chose between interferon or DLI.
Future trials will examine the addition of very low dose DLI to treat patients who have achieved a complete cytogenetic, but not molecular response, to interferon. If this approach proves effective without significant life-threatening toxicities, a randomized trial of interferon induction of a minimal residual disease state followed by DLI versus DLI alone might be warranted.
ACKNOWLEDGMENT
The authors acknowledge and thank Catherine Morgan (cytogenetics lab) and Arthur Lee (PCR lab) for their years of involvement in these studies. We are indebted to Dr Judith Prestifilippo of Hoffmann-LaRoche who has been instrumental in providing on-going support for these studies.
Supported in part by Grant Nos. CA16448 and CA18029 from the National Cancer Institute (Bethesda, MD), Department of Health and Human Services (Washington, DC), and an educational grant from Hoffmann-LaRoche (Nutley, NJ).
Address reprint requests to Celestia S. Higano, MD, University Hospital, Box 356043, 1959 NE Pacific St, Seattle, WA 98195.