Abstract
Tissue factor pathway inhibitor (TFPI) is a multivalent Kunitz-type proteinase inhibitor that directly inhibits factor Xa and, in a factor Xa–dependent fashion, produces feedback inhibition of the factor VIIa/TF catalytic complex responsible for the initiation of coagulation. To further define the physiologic role of TFPI, gene-targeting techniques were used to disrupt exon 4 of the TFPI gene in mice. This exon encodes Kunitz domain-1 of TFPI, which is required for factor VIIa/TF inhibition. In mice heterozygous for TFPI gene-disruption, TFPIK1(+/−), an altered form of TFPI lacking Kunitz domain-1, circulates in plasma at a concentration ∼40% that of wild-type TFPI. TFPIK1(+/−) animals have plasma TFPI activity ∼50% that of wild-type mice, based on a functional assay that measures factor VIIa/TF inhibition, and have a normal phenotype. Sixty percent of TFPIK1(−/−) mice die between embryonic days E9.5 and E11.5 with signs of yolk sac hemorrhage. The extent of structural abnormalities within the yolk sac vascular system appears to mirror the condition of the embryo, suggesting that the embryonic and extra-embryonic tissues are both responding to same insult, presumably circulatory insufficiency. Organogenesis is normal in TFPIK1 null animals that progress beyond E11.5, but hemorrhage, particularly in the central nervous system and tail, is evident during later gestation and none of the TFPIK1(−/−) mice survive to the neonatal period. The presence of immunoreactive fibrin(ogen) in the liver and intravascular thrombi is consistent with the notion that unregulated factor VIIa/TF action and a consequent consumptive coagulopathy underlies the bleeding diathesis in these older embryos. Human TFPI-deficient embryos may suffer a similar fate because an individual with TFPI deficiency has not been identified.
TISSUE FACTOR pathway inhibitor (TFPI) is a multivalent Kunitz-type proteinase inhibitor that regulates the initiation of coagulation by producing factor Xa–mediated feedback inhibition of the factor VIIa/TF catalytic complex.1 The concentration of TFPI in plasma is low (∼2 nmol/L) and much of the circulating TFPI is associated with plasma lipoproteins (LDL > HDL ≫ VLDL).2 Platelets carry approximately 10% of the total blood TFPI and release their TFPI after stimulation with thrombin and other agonists.3 The infusion of heparin in vivo increases circulating levels of TFPI in plasma twofold to fourfold and the source of this additional TFPI is thought to be the endothelium where TFPI may be bound to surface glycosaminoglycans.4 5
The TFPI molecule has an acidic amino-terminus followed by three tandem Kunitz-type proteinase inhibitory domains and a basic carboxy-terminus.6 The second Kunitz-type domain binds and inhibits factor Xa and the first Kunitz domain binds and inhibits factor VIIa in a quaternary inhibitory complex containing factor Xa-TFPI-factor VIIa/TF.7 A proteinase inhibitory role for the third Kunitz-type domain has not been established. Positively charged residues in the third Kunitz-type domain and the carboxy-terminal tail of TFPI mediate its binding to heparin and are required for the binding of TFPI to cell surfaces, including the endothelium.8,9 Recent studies suggest that TFPI bound at the surface of cells mediates the internalization and degradation of factor Xa and the transfer of the factor VIIa/TF complex to caveolae.10 11
Immuno-depletion of endogenous TFPI sensitizes rabbits to the disseminated intravascular coagulation (DIC) induced by TF or endotoxin infusion,12,13 but does not affect the coagulopathy produced by the infusion of a complex of factor Xa and phospholipids,14 suggesting that the major role of TFPI is regulation of factor VIIa/TF activity. Conversely, the infusion of high, therapeutic concentrations of TFPI ameliorates the intravascular coagulation induced by TF in rabbits and prevents mortality in a baboon model of Escherichia coli sepsis.15,16 Although TFPI deficiency might be expected to cause a prothrombotic phenotype, no individual with TFPI has yet been identified. The low plasma levels of TFPI found in abetalipoproteinemic patients appear to simply reflect the absence of low-density lipoprotein (LDL), a carrier of TFPI in plasma.5 The total available TFPI in these patients, as estimated by plasma TFPI levels after heparin infusion, is similar to that of normal individuals. Further, abetalipoproteinemic patients do not appear to have an increased risk of thrombosis.
To better define the physiological role of TFPI, we have used gene targeting in mouse embryonic stem cells to create mice with a disrupted TFPI gene. Homozygous TFPI gene disruption is associated with intrauterine mortality and signs of a hemorrhagic diathesis.
MATERIALS AND METHODS
Animals.Mice were kept in micro-isolator cages on a 12-hour day/night cycle and were fed regular chow. Intraperitoneal Avertin (2.5%, 0.015 mL/g) was used as anesthetic for surgical procedures.
Construction of TFPI gene-targeting vector.An ∼15-kb segment of DNA containing exons 4 and 5 of the mouse TFPI gene was isolated from a 129Sv lambda fix II genomic DNA library (Stratagene, La Jolla, CA) using a full-length mouse TFPI cDNA as probe and a 9-kb Not I-Spe I fragment of the genomic DNA was subcloned into pBluescript II KS+ (Stratagene) (see Fig 1). A 0.7-kb Bst11071 fragment containing portions of exon 4 and intron D was deleted and replaced by a 1.5-kb PGK-neomycin phosphotransferase expression cassette (neo) and a 1.8-kb HSV-thymidine kinase expression cassette (tk) was inserted 3′ to the DNA segment at a Xho I site within the pBluescript multiple cloning sequence (Fig 1). The structure of the targeting vector was confirmed by both restriction enzyme mapping and sequencing of ligation junctions.
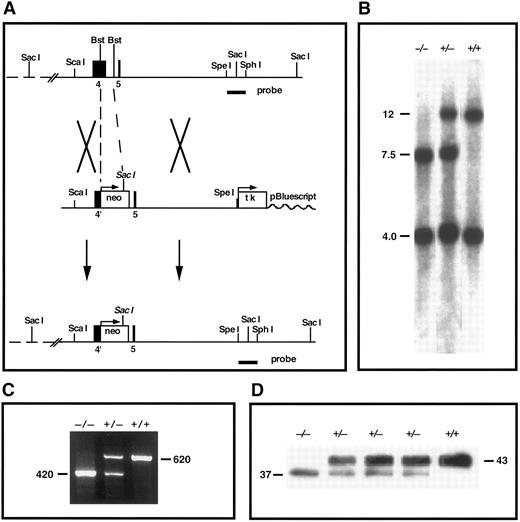
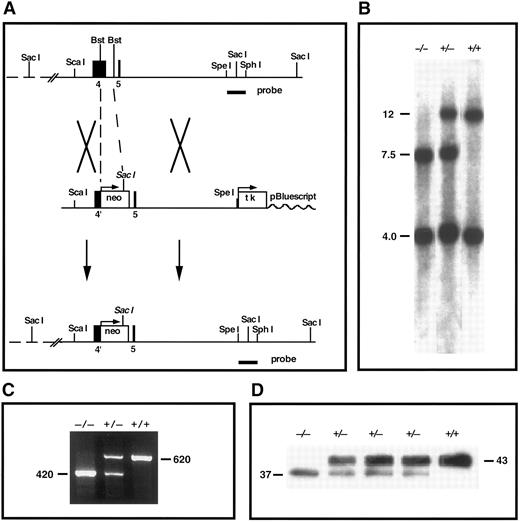
TFPI gene-disruption in mice. (A) TFPI replacement construct and partial restriction map of the endogenous locus. Exon 4 encodes the Kunitz-1 domain of mouse TFPI. neo, neomycin phosphotransferase gene; tk, thymidine kinase gene; Bst, Bst1107I; probe, TFPI-specific probe used in Southern analysis. (B) Southern blot confirmation of TFPI gene disruption. The probe indicated in (A) was used to probe Sac I digests of tail DNA from mutant and nonmutant mice (genotype listed). This probe detects DNA fragments of 12 and 4 kb in the wild-type allele and fragments of 7.5 and 4 kb in the disrupted allele. (C) RT-PCR demonstration of the production of two TFPI mRNAs in TFPIK1(+/−) mice. One PCR product (620 bp) encodes wild-type TFPI, the other (420 bp) encodes TFPIdesK1 , an altered form of TFPI in which the first Kunitz domain (encoded by exon 4 of the TFPI gene) has been deleted. (D) Western blot analysis of plasma TFPI in wild-type and mutant mice (genotype listed). The protein band at 43,000 MW represents wild-type TFPI; an additional form of TFPI (TFPIdesK1 ) at 37,000 MW is apparent in the plasma from TFPIK1(+/−) mice and a TFPIK1(−/−) mouse.
TFPI gene-disruption in mice. (A) TFPI replacement construct and partial restriction map of the endogenous locus. Exon 4 encodes the Kunitz-1 domain of mouse TFPI. neo, neomycin phosphotransferase gene; tk, thymidine kinase gene; Bst, Bst1107I; probe, TFPI-specific probe used in Southern analysis. (B) Southern blot confirmation of TFPI gene disruption. The probe indicated in (A) was used to probe Sac I digests of tail DNA from mutant and nonmutant mice (genotype listed). This probe detects DNA fragments of 12 and 4 kb in the wild-type allele and fragments of 7.5 and 4 kb in the disrupted allele. (C) RT-PCR demonstration of the production of two TFPI mRNAs in TFPIK1(+/−) mice. One PCR product (620 bp) encodes wild-type TFPI, the other (420 bp) encodes TFPIdesK1 , an altered form of TFPI in which the first Kunitz domain (encoded by exon 4 of the TFPI gene) has been deleted. (D) Western blot analysis of plasma TFPI in wild-type and mutant mice (genotype listed). The protein band at 43,000 MW represents wild-type TFPI; an additional form of TFPI (TFPIdesK1 ) at 37,000 MW is apparent in the plasma from TFPIK1(+/−) mice and a TFPIK1(−/−) mouse.
Targeted TFPI gene disruption in embryonic stem cells.The targeting vector was linearized with Not I and introduced by electroporation into RW-4 (R. Wesselschmidt and T. Ley, Washington University, St Louis, MO) and R-1 (A. Nagy, University of Toronto, Toronto, Ontario, Canada) 129 mouse embryonic stem (ES) cells. The transfected ES cells were grown on irradiated neomycin-resistant mouse embryonic fibroblasts and selected with G418 (400 μg/mL; GIBCO/BRL, Grand Island, NY) and gancyclovir (2 μmol/L; Syntex, Palo Alto, CA). After 7 days, surviving clones were tested for homologous recombination at a TFPI allele by Southern analysis (see below). Five of 250 RW-4 and 1 of 50 R-1 selected ES cell clones were positive for targeted TFPI gene disruption.
Generation of TFPI-deficient mice.C57B1/6 blastocysts (3.5 days postcoitus) were injected with ES cells from one RW-4 clone or the R-1 clone and transferred to the uterine horns of 2.5 days postcoitus pseudopregnant recipient Swiss Webster mice. Male progeny greater than 80% chimeric by coat color were mated with C57B1/6 mice and germline transmission of the disrupted TFPI gene was confirmed by Southern analysis of tail DNA. Heterozygous TFPI gene-disrupted mice were interbred to produce animals with homozygous TFPI gene disruption. The mouse lines derived from the separate targeted RW-4 and R-1 ES clones were studied independently.
Timed matings.Heterozygous female mice in estrus were placed with heterozygous males in the evening. Noon on the day of vaginal plug detection was defined as embryonic day 0.5 (E0.5). At various times of gestation, females were killed and the embryos harvested. Embryos and yolk sacs were carefully dissected free of maternal tissue. One was used for genotyping by Southern analysis and the other was fixed in 4% phosphate-buffered formaldehyde. Fixed tissue was then dehydrated, paraffin embedded, and sectioned according to standard protocols. Sections were stained with hematoxylin and eosin (H&E).
Southern blot analysis of genomic DNA.A 1.4-kb Spe I-Sph I fragment of TFPI genomic DNA 3′ of the targeting construct was used for Southern analysis (Fig 1). After digestion of genomic DNA with Sac I, the probe recognizes bands of 12 and 4 kb in the wild-type TFPI allele and bands of 7.5 and 4 kb in the disrupted TFPI allele.
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA was isolated from day 14.5 embryos using TRIZOL (GIBCO/BRL, Gaithersburg, MD). First-strand cDNA was synthesized by SUPERSCRIPT II reverse transcriptase (GIBCO/BRL) using a primer located at the junction of exons 6 and 7 of the mouse TFPI gene (GAG TGG ACT GGA TTC TCA CAG ATC). Subsequent PCR used the RT primer and a forward primer located at the junction of exons 1 and 2 (TGT ATC ACT TTC GGG ACC TGT CTC). PCR products of 620 and 420 bp were detected in samples from animals with heterozygotic TFPI gene-disruption (Fig 1). These were separated on low-melting-point agarose, the target bands isolated from the gel, and the DNAs cloned into pGEM-T (Promega, Madison, WI) for sequencing (Fig 1). The oligonucleotide sequence of the 620-bp PCR product is that of native TFPI cDNA. In the 420-bp PCR product, exon 3 DNA (encoding the amino-terminus of TFPI) is linked in frame to exon 5 (encoding the polypeptide between Kunitz domains 1 and 2) and exon 4 (encoding Kunitz domain 1) is omitted (data not shown).
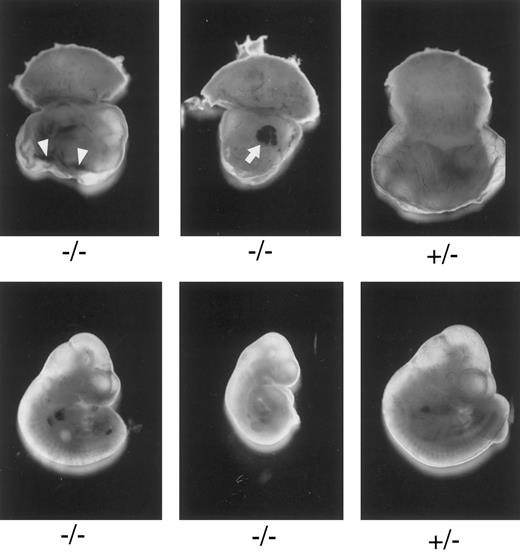
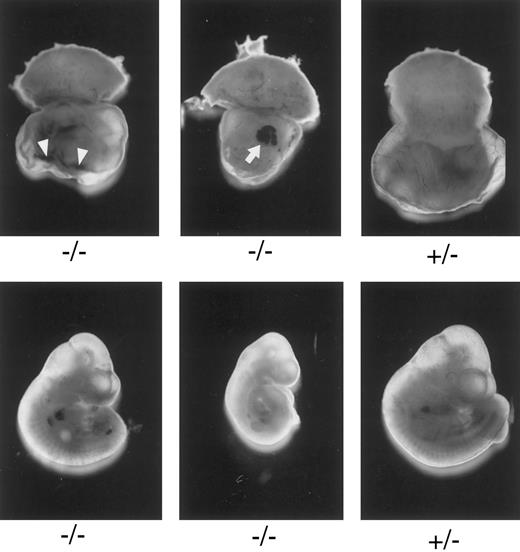
Macroscopic examination of E10.3 embryos. TFPIK1 genotypes are indicated. Arrowheads, intra-yolk sac hemorrhage with red blood cells in yolk sac folds; arrow, blood “lakes” in yolk sac. Note that yolk sac hemorrhage can be found with a near normal embryo (left, bottom), suggesting that hemorrhage and concomitant circulatory collapse precede the wasting and ultimate death of the embryo.
Macroscopic examination of E10.3 embryos. TFPIK1 genotypes are indicated. Arrowheads, intra-yolk sac hemorrhage with red blood cells in yolk sac folds; arrow, blood “lakes” in yolk sac. Note that yolk sac hemorrhage can be found with a near normal embryo (left, bottom), suggesting that hemorrhage and concomitant circulatory collapse precede the wasting and ultimate death of the embryo.
Western blot analysis.Mouse plasma samples (30 μL) were diluted with rabbit plasma (220 μL) and rocked with rabbit-antimouse TFPI IgG linked to Affigel 10 (10 mg/mL) (50 μL of 50% slurry). After 1 hour, the Affigel beads were isolated by centrifugation, washed with buffer, and the bound TFPI eluted by incubation at 100°C with 4% sodium dodecyl sulfate (SDS) (50 μL). Samples were applied to 15% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose for blotting with rabbit-antimouse TFPI antisera (1:300 dilution). The SuperSignal CL-HRP Substrate System (Pierce, Rockford, IL) was used for detection and a Molecular Dynamics Personal Densitometer (Sunnyvale, CA) was used to scan and quantify the images. Plasma from an animal with homozygous TFPI gene disruption was obtained from an 8-week-old mouse that had been derived by breeding animals doubly heterozygous for TFPI gene disruption and a transgene containing the transferrin promoter directing the expression of a protein called XK1. XK1 is a human chimeric protein (30,000 molecular weight [MW]) containing the amino-terminal light chain of factor X and the Kunitz-1 domain of TFPI and directly inhibits factor VIIa/TF in both the human and mouse systems.17 Human XK1 is not recognized by the rabbit-antimouse TFPI antisera.
Antifibrin(ogen) immunohistochemical staining.Slides were deparaffinized in xylene (10 minutes × 2), transferred to 100% alcohol (2 minutes × 2), and incubated for 10 minutes in 3% H2O2 in methanol to block endogenous peroxidase activity. After rinsing three times, the slides were incubated successively with blotto (3% dry milk, 30 minutes, room temperature), goat-antimouse fibrin (ogen) IgG (Nordic Immunology, Tilburg, The Netherlands, distributed by Accurate Chemical Co, Westbury, NY), or control goat IgG (4 μg/mL, overnight at 4°C), and developed using rabbit-antigoat IgG-horseradish peroxidase conjugate (1:5,000 dilution) and AEC (3-amino-9-ethylcarbazole) per the manufacturer's instructions (Sigma Chemical Co, St Louis, MO).
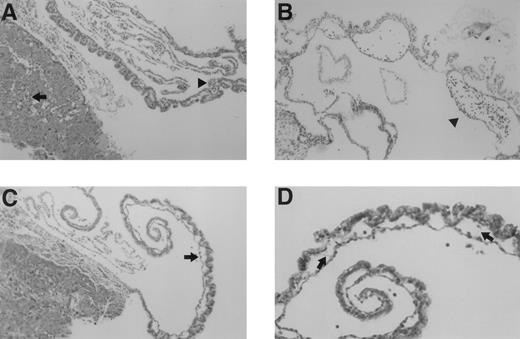
Microscopic examination of yolk sac and placenta of E10.5 TFPIK1(−/−) embryos. (A) Normal-appearing placenta and yolk sac from a normal appearing embryo. Nucleated embryonic erythrocytes are present in placenta (arrow) and yolk sac (arrowhead) vessels. (B) Yolk sac of a severely growth and developmentally retarded embryo displaying disruption and degeneration of yolk sac tissues. Arrowhead denotes blood cells within a broad space between endoderm and mesoderm layers, perhaps the microscopic equivalent of a macroscopic blood “lake” (Fig 2). (C) Placenta and yolk sac of modestly growth retarded embryo. Note lack of nucleated erythrocytes in placenta and yolk sac vasculature and the lateral expansion of yolk sac vessels (arrow). (D) Higher magnification of yolk sac shown in (C). Arrows point to thin acellular septae connecting the endodermal and mesodermal layers.
Microscopic examination of yolk sac and placenta of E10.5 TFPIK1(−/−) embryos. (A) Normal-appearing placenta and yolk sac from a normal appearing embryo. Nucleated embryonic erythrocytes are present in placenta (arrow) and yolk sac (arrowhead) vessels. (B) Yolk sac of a severely growth and developmentally retarded embryo displaying disruption and degeneration of yolk sac tissues. Arrowhead denotes blood cells within a broad space between endoderm and mesoderm layers, perhaps the microscopic equivalent of a macroscopic blood “lake” (Fig 2). (C) Placenta and yolk sac of modestly growth retarded embryo. Note lack of nucleated erythrocytes in placenta and yolk sac vasculature and the lateral expansion of yolk sac vessels (arrow). (D) Higher magnification of yolk sac shown in (C). Arrows point to thin acellular septae connecting the endodermal and mesodermal layers.
Other methods.TFPI functional assay was performed as previously described18 except serum samples were used rather than plasma samples. The mean serum TFPI activity of wild-type mice was arbitrarily assigned a value of 100%. Platelet counts were done by phase contrast. Fibrinogen immunoassay was performed using goat-antimouse fibrinogen IgG (Nordic Immunology) and an enzyme-linked immunosorbent assay (ELISA) format. The mean fibrinogen concentration of wild-type mice was arbitrarily assigned a value of 100%.
RESULTS
Disruption of the mouse TFPI gene.A gene-targeting vector in which a portion of exon 4 (encoding Kunitz domain 1) and intron D in the mouse TFPI gene is replaced with the neomycin phosphotransferase gene was introduced into embryonic stem cells by electroporation and 2% of selected clones tested positive for appropriate homologous recombination at the TFPI locus by Southern analysis (Fig 1). Two mouse lines derived from separate embryonic stem cells carrying the altered TFPI gene were produced and studied independently. The characteristics of these mouse lines were indistinguishable and the results of studies in both lines have been combined for this report.
The intron-exon boundaries in the TFPI gene are all of the same type19 and, because of alternative mRNA splicing, mice carrying the disrupted TFPI allele express a protein product in which Kunitz domain 1 is deleted (TFPIdesK1 ) (Fig 1). Based on densitometric analysis of Western blots of plasma from animals heterozygous for TFPI gene disruption, the expression of the altered form of TFPI by the disrupted allele is 41% ± 7% (n = 6) that of the expression of wild-type TFPI from the normal TFPI allele (Fig 1). The TFPIdesK1 protein binds factor Xa on ligand blot (not shown), but cannot produce factor VIIa/tissue factor inhibition. The functional activity of TFPI in the serum of TFPIK1(+/+) (n = 9) and TFPIK1(+/−) (n = 11) littermate mice measured by an assay based on factor VIIa/TF inhibition is 100% ± 17% and 46% ± 7%, respectively.
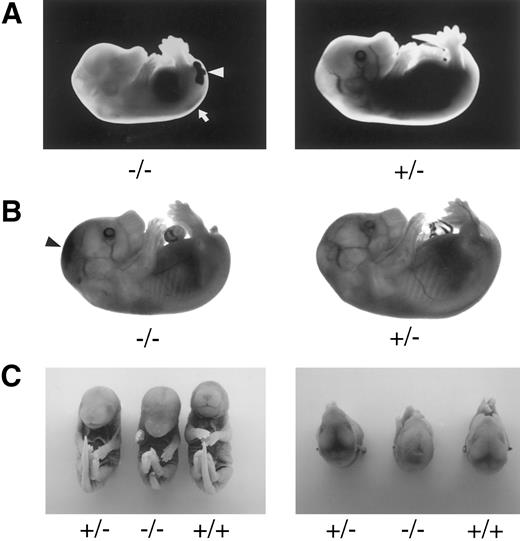
Late-gestation embryos. TFPIK1 genotypes are indicated. (A) Pale, stunted TFPIK1(−/−) E14.5 embryo with hemorrhage in the central canal of the spinal cord (arrow) and tail (arrowhead). (B) TFPIK1(−/−) E16.5 embryo with intercranial hemorrhage (arrowhead) and short, kinked tail; note absence of blood in vasculature. (C) E18.5 embryos. Note short tail, paucity of blood in cranial venous sinuses, and sunken cranial fontenelle in the TFPIK1(−/−) embryo. Microscopic sections of this animal showed intercranial hemorrhage and degeneration of brain tissue.
Late-gestation embryos. TFPIK1 genotypes are indicated. (A) Pale, stunted TFPIK1(−/−) E14.5 embryo with hemorrhage in the central canal of the spinal cord (arrow) and tail (arrowhead). (B) TFPIK1(−/−) E16.5 embryo with intercranial hemorrhage (arrowhead) and short, kinked tail; note absence of blood in vasculature. (C) E18.5 embryos. Note short tail, paucity of blood in cranial venous sinuses, and sunken cranial fontenelle in the TFPIK1(−/−) embryo. Microscopic sections of this animal showed intercranial hemorrhage and degeneration of brain tissue.
The growth and development of heterozygotic TFPIK1(+/−) animals is indistinguishable from that of their wild-type TFPIK1(+/+) littermates. TFPIK1(+/−) mice can produce and sustain multiple litters and suffered no untoward effects from the tail transection used to obtain tissue for genotyping. Blood and plasma levels of the following hematological parameters in TFPIK1(+/+) (n = 8) and TFPIK1(+/−) (n = 8) mice were as follows: hematocrit, 54% ± 2% and 55% ± 2%; platelet count, 532,000 ± 87,000 and 565,000 ± 97,000; and fibrinogen, 100% ± 13% and 92% ± 19%, respectively. In the absence of additional challenge, a 50% level of TFPI-mediated inhibition of factor VIIa/TF appears compatible with normal survival.
Characterization of TFPIK1(−/−) mice.Of 233 pups born following the mating of heterozygotic TFPIK1(+/−) mice, 35% were TFPIK1(+/+) and 65% were TFPIK1(+/−) (Table 1). To examine the phenotype of TFPIK1(−/−) mice, embryos were collected at various times of gestation after matings between TFPIK1(+/−) mice. TFPIK1(−/−) embryos were indistinguishable from TFPIK1(+/−) and TFPIK1(+/+) at embryonic day 8.5 (E8.5) (data not shown). Between E9.5 and E11.5, however, many of the TFPIK1(−/−) concepti displayed intra-yolk sac hemorrhage and a paucity of blood in yolk sac vessels (Fig 2). The embryos contained within these yolk sacs were usually pale and growth retarded, and occasionally had pericardial effusions containing extravasated red blood cells.
Histological sections at this period show a spectrum of abnormalities in the yolk sac and placenta of TFPIK1(−/−) embryos. Vascular abnormalities in these extra-embryonic tissues appear to increase in concert with progressive growth retardation in the embryo. Normal-appearing TFPIK1(−/−) embryos had normal-appearing yolk sacs (Fig 3A). Pallor and moderate growth retardation in embryos were associated with decreased quantities of fetal red blood cells in the yolk sac and placental vessels and lateral expansion of capillaries in the visceral yolk sac due to the loss of contact points between the endodermal and mesodermal cell layers with the apparent fusion of adjacent vessels (Fig 3C). At times, fine septae connecting the endoderm and mesoderm remain at apparent sites of previous contact (Fig 3D). Concepti with severely affected embryos have marked abnormalities of the yolk architecture, with broad separation of yolk sac endodermal and mesodermal cell layers and signs of necrosis (Fig 3B).
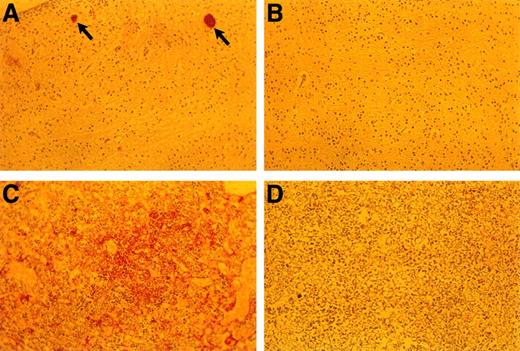
The incidence of yolk sac hemorrhage is not absolute and ∼40% of the TFPIK1(−/−) embryos survive beyond E10.5 (Table 1). Organ development in these embryos, including the heart, vasculature, and hepatic hematopoiesis, is normal, but TFPIK1(−/−) mice frequently have short tails (Fig 4). Hemorrhage, particularly in the head, spine, and tail, with accompanying pallor and growth retardation was a striking feature of the older TFPIK1(−/−) animals (Fig 4). Deposition of immunoreactive fibrin(ogen) within the hepatic interstitia20 and rare intravascular thrombosis were detectable in >E12.5 TFPIK1(−/−) embryos (Fig 5).
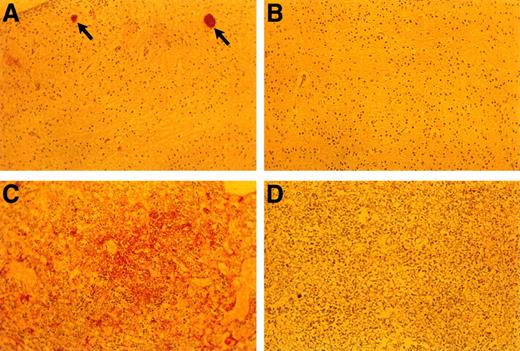
Anti-fibrin(ogen) staining of E18.5 embryonic tissues. (A) Brain of TFPIK1(−/−) embryo. Arrows denote two small vessels with apparent fibrin thrombi. (B) Brain of TFPIK1(+/−) embryo. (C) Diffuse anti-fibrin(ogen) staining within the interstitia of the liver of a TFPIK1(−/−) embryo. (D) Liver of TFPIK1(+/−) embryo.
Anti-fibrin(ogen) staining of E18.5 embryonic tissues. (A) Brain of TFPIK1(−/−) embryo. Arrows denote two small vessels with apparent fibrin thrombi. (B) Brain of TFPIK1(+/−) embryo. (C) Diffuse anti-fibrin(ogen) staining within the interstitia of the liver of a TFPIK1(−/−) embryo. (D) Liver of TFPIK1(+/−) embryo.
DISCUSSION
The earliest abnormalities observed with TFPI deficiency are the yolk sac hemorrhage and loss of blood in the yolk sac vasculature which are detectable at E9.5. These changes are usually found in association with a pale embryo with severe growth and developmental retardation. Yolk sac hemorrhage can be found with a near-normal embryo (Fig 2), however, suggesting that hemorrhage and concomitant circulatory collapse precede and lead to the wasting and ultimate death of the embryo. The extent of the histological yolk sac abnormalities appears to mirror the condition of the embryo, suggesting that the embryonic and extra-embryonic tissues of these TFPIK1(−/−) concepti are responding to the same insult (Fig 3). The blood “lakes” (Fig 2) and thin acellular septae connecting the visceral endoderm and mesoderm of the yolk sac (Fig 3D) that are found in TFPIK1(−/−) concepti have also been noted in TF-deficient embryos,21 but the TF activity of TFPIK1(−/−) whole embryos and their derived embryonic fibroblasts is equivalent to that of TFPIK1(+/+) animals (data not shown).
Whether a consumptive coagulopathy due to unfettered factor VIIa/TF action contributes to the loss of vascular integrity in early gestation TFPIK1(−/−) embryos is not clear. The challenge to the vascular integrity of the extra-embryonic circulation between E8.5-E11.5 appears to be substantial. It is during this period that the blood islands fuse to produce the visceral yolk sac vascular system, the allantois and chorion fuse to link the embryo to the placenta, and the intravascular pressure increases. A significant proportion of TF-deficient (85%)22,23 and factor V–deficient (50%)24 mice die during this period, suggesting that the initiation of coagulation and the generation of thrombin is important, though not essential, for the maintenance of the embryo at this stage of development. Fibrinogen-deficient mice produced by disrupting the gene for one (α) of the three fibrinogen polypeptide chains (α, β, γ), on the other hand, are born in normal numbers.25 Whether trace quantities of β/γ fibrinogen, another embryonic protein(s), or maternally derived fibrinogen compensate for the lack of embryonic fibrinogen during early embryogenesis in these animals is not known. Such putative maternal transfer of other soluble proteins, namely factor V and TFPI, is clearly insufficient to completely rescue the respective deficient embryos from mid-gestation mortality. Alternatively, thrombin and/or other agents generated through the activation of coagulation may affect vascular integrity through mechanisms unrelated to classical hemostasis and the clotting of fibrinogen. The observation that 50% of thrombin receptor gene deleted mice die between E9.0 and E10.0 is perhaps consistent with this latter scenario.26
Organogenesis, including cardiac and vascular development, appear normal in the proportion (40%) of TFPIK1(−/−) animals that survive to later gestational dates. Thus, TFPI is not essential for the development of a specific tissue. The detection of apparent hepatic fibrin deposition and intravascular thrombi is consistent with the notion that unregulated factor VIIa/TF action and a consequent consumptive coagulopathy underlies the bleeding diathesis in these older embryos (Fig 5). Interestingly, while less than 15% of TF(−/−) embryos progress to greater than E10.5, the few that are present at later gestational dates do not show signs of bleeding.23 Mice with fibrinogen deficiency,25 factor VIII deficiency,27 or thrombocytopenia28 suffer hemorrhagic symptoms postnatally but are born in normal numbers. Half of factor V–deficient mice also survive to birth.24 The greater apparent severity of the TFPIK1(−/−) hemorrhagic phenotype may be due to the combination of plasma and cellular (eg, thrombocytopenia) hemostatic defects associated with disseminated intravascular coagulation.
These studies show that the Kunitz-1 domain of TFPI is essential for embryonic survival in mice. Whatever the cause(s) of the loss of vascular integrity in early and late gestation TFPIK1(−/−) mice, intrauterine lethality appears to be the result of a hemorrhage and circulatory insufficiency. Human TFPI-deficient embryos may suffer a similar fate because an individual with TFPI deficiency has not been identified.
ACKNOWLEDGMENT
We thank Dr X. Han for performing the plasma fibrinogen immunoassays and T. Lewis for preparing this manuscript.
Supported by Grant No. R01 HL 34462 from the National Institutes of Health.
Address reprint requests to George J. Broze, Jr, MD, Barnes-Jewish Hospital, 216 S Kingshighway Blvd, St Louis, MO 63110.