Abstract
The requisite initial reaction for in vivo thrombus formation in flowing blood is platelet adhesion to the exposed surface of the extracellular matrix. The contribution of von Willebrand factor (vWF ) in plasma and glycoprotein (GP) Ib on the platelet membrane to platelet adhesion has been well-documented. We have recently developed a procedure (the “flow adhesion assay”) for measuring platelet adhesion under flow conditions that allowed us to characterize platelet adhesion to a collagen-coated surface. Here, we apply our method to analyze platelet adhesion to a vWF-coated surface to determine how this might differ from adhesion to a collagen-coated surface. Platelet adhesion to the vWF-coated surface was monitored as the linear increase in the area occupied by adherent platelets. The fluorescence image showed that platelets adhering to the vWF surface were mainly single platelets, and if any were present, the platelet aggregates were small, this being the primary difference from the adhesion to a collagen surface, where adherent platelets were mostly in aggregates. The flow adhesion assay detected the movement of platelets on the vWF surface, suggesting the reversible binding of vWF with platelets. The velocity of the platelets increased at higher shear rates or at lower vWF densities on the surface. Treatment of the vWF-coated surface with the aggregating agent botrocetin before initiation of blood flow increased platelet adhesion while dramatically decreasing the velocity of platelet movement. The present observations on the adhesion of platelets to the vWF-pretreated collagen surface and measurements of the velocity of platelets moving on the collagen surface suggest that the first interaction on the collagen-coated surface is the binding of vWF molecules to the collagen surface. This small number of vWF molecules would serve to attract and slow platelets flowing near the surface. This would facilitate the actual adhesion to the collagen surface that is mainly generated by the interaction between platelet collagen receptors, including GP Ia/IIa and GP VI, with collagen.
THE FIRST AND critical reaction of in vivo thrombus formation in flowing blood is the adhesion of platelets to the exposed surface of the extracellular matrix. Despite its importance, the mechanism by which platelets adhere to the extracellular matrix under flow conditions has not been satisfactorily resolved. In platelet adhesion under flow conditions, the interaction between von Willebrand factor (vWF ) in plasma and its receptor glycoprotein Ib (GP Ib) on platelet surfaces was indicated to be the primary one, especially under high-shear conditions, on the basis of perfusion studies, a method first introduced by Baumgartner's group.1,2 Although there are detailed analyses of the interaction between GP Ib and vWF in vitro and the sites in vWF and GP Ib that contribute to this interaction have been identified,3 the nature and contribution of the GPIb-vWF interaction to platelet adhesion under flow conditions is much less well understood. The main question is what is the mechanism through which platelets adhere to the immobilized vWF under flow conditions, although normal platelets do not bind human vWF in solution. There are two hypothesis: vWF may undergo a conformational change after immobilization on the surface to enhance its binding affinity to platelets, or alternatively, immobilization of vWF may itself increase the affinity to platelets because vWF is a multimeric protein with very high molecular weight.4 Recently, Goto et al5 proposed another hypothesis that GP Ib on the platelet surface interacts reversibly with vWF and shear stress induces a permanent interaction between vWF and GP IIb/IIIa after the initial binding of GP Ib with vWF. This proposal was also supported by a perfusion study by the same group.6 In the latter hypothesis, platelets would interact with vWF in the flowing blood, but the dissociation rate of this interaction is high, so the binding becomes reversible. Another interaction is required to arrest platelets on the surface.
In a previous study,7 we developed a new perfusion device based on the method of Hubbell and McIntire,8 and analyzed platelet adhesion on a collagen-coated surface under flow conditions. This adhesion was biphasic, being characterized by platelets that adhered transiently at first, a phenomenon we called “temporary arrest.” We suggested that the interaction between GP Ib and vWF would be involved in this temporary arrest. In this study, we analyzed platelet adhesion on a vWF-coated surface and compared it with adhesion on the collagen-coated surface using our perfusion method. Our data supported the hypothesis proposed by Ruggeri's group that the interaction between GP Ib on the platelet surface and vWF is reversible. We further analyzed this reaction using reagents that inhibit platelet aggregation and botrocetin that induces platelet aggregation by interacting with vWF. By comparing adhesion on the collagen-coated surface with that on the vWF-coated surface, we showed that the first reaction of the adhesion process on the collagen surface, the temporary arrest, is the adhesion of vWF adsorbed on the collagen surface. The prominent difference in the pattern of platelet adhesion between the collagen surface and the vWF surface, together with our previous results,7 suggests that the formation of platelet aggregates on the collagen surface is induced by the interaction of arrested platelets with the collagen surface and not by the interaction with adsorbed vWF.
MATERIALS AND METHODS
Blood samples. Whole blood was drawn from the cubital vein of a healthy volunteer into 0.1 vol 3.8% sodium citrate. The blood was incubated with 5 μmol/L mepacrine (Sigma, St Louis, MO) for 30 to 120 minutes at room temperature and used for the flow adhesion assay (fluorescent adhesion assay). The whole blood was directly used for the parallel-plate perfusion assay (fixing adhesion assay) after leaving it for 1 hour at room temperature.
Materials. vWF was purified from outdated blood according to the method of Thorell and Blomback.9 vWF was purified by precipitating it in the presence of 1.55 mol/L NaCl and 2 mol/L glycine, followed by gel filtration over Sephacryl S400 HR (Pharmacia Biotech, Tokyo, Japan). The obtained vWF samples were applied to gelatin-Sepharose (Pharmacia Biotech)10 and antifibrinogen–coupled Sepharose columns to remove the remaining fibronectin and fibrinogen contaminants, respectively. Antifibrinogen monoclonal antibody, IF-1, was coupled to an NHS-activated HiTrap column (Pharmacia Biotech) and used for immunoadsorption of fibrinogen according to the original report.11 The obtained vWF solution was concentrated by ultrafiltration using an Omegacell (Filtron Technology Corp, Clinton, MA) and then dialyzed against HEPES-Tyrode buffer containing 136 mmol/L NaCl, 2.7 mmol/L KCl, 0.42 mmol/L NaH2PO4 , 12 mmol/L NaHCO3 , 5.5 mmol/L glucose, and 5 mmol/L HEPES, pH 7.4. The residual contents of fibrinogen and fibronectin in the final preparation of vWF were determined by the ELISA method, which showed the presence of a trace amount of fibrinogen and fibronectin, less than 0.1% of the total protein. Botrocetin was a kind gift from Dr T. Morita, Meiji College of Pharmacy, Tokyo.12
The anti-GP IIb/IIIa monoclonal antibody P2 was purchased from Immunotech International (Marseilles, France), and the anti-vWF monoclonal antibody AJvW-2 was kindly provided by Dr R. Yoshimoto, Central Research Laboratories of Ajinomoto Co, Tokyo.13 The characteristics of the anti-GP Ib monoclonal antibody NNKY5-5 were previously reported.14,15 The Gly-Arg-Gly-Asp-Ser (GRGDS) peptide was prepared in this laboratory.7 Bovine type III collagen was obtained from Koken (Tokyo, Japan).
Measurement of the amount of adsorbed proteins on the glass surface. Siliconized, ethanol-treated or untreated (“native”) cover glasses (240 × 500 mm; Takahashi Giken, Tokyo, Japan) were used for these experiments. The siliconized cover glasses were prepared as follows: they were treated with trifluoroacetic acid (Applied Biosystems, Tokyo, Japan) for 2 hours, dried under a vacuum, and then reacted with 0.2% 3-aminopropyltriethoxysilane (Pierce, Rockford, IL) in water for 3 hours at room temperature. After washing the cover glasses eight to 10 times with acetone, they were dried in vacuo for 45 minutes.16 To prepare ethanol-treated glasses, the cover glasses were washed three times in ethanol and then dried under vacuum. Cover glasses of each type were individually cut into five pieces and weighed, and the area of each piece was calculated from its weight.
vWF and collagen were radiolabeled by NaI125 (Amersham Life Science, Tokyo, Japan) using Iodobeads (Pierce) according to the manufacturer's instructions. Protein concentrations of the radiolabeled proteins were determined by the bicinchoninic acid protein assay procedure with the corresponding cold proteins as standards.17 The calculated specific radioactivities were 3.09 × 105 cpm/μg and 2.94 × 105 cpm/μg for vWF and collagen, respectively.
Different concentrations of 125I-labeled vWF and collagen solutions were layered over each glass piece and incubated overnight in the cold. Nonadsorbed proteins were removed by washing the glasses two times with HEPES-Tyrode buffer containing 2% bovine serum albumin (BSA), followed by two washes with HEPES-Tyrode buffer without BSA. After drying the glasses in the hood, the radioactivity of each glass piece was measured in a Wallac 1282 gamma counter (LKB, Bromma, Sweden); the amount of adsorbed protein per square millimeter of surface was calculated for each cover glass piece.
Perfusion studies. The details of the apparatus for measuring platelet adhesion by the fluorescent adhesion assay were described previously.7 Mepacrine-loaded blood was passed through the perfusion chamber, and the adherent platelets were monitored by fluorescent microscopy. The fluorescent images were recorded by a videocamera, and fluorescent signals were processed by computer-assisted analyses to quantify the extent of platelet adhesion and of platelet movement. Usually, fluorescent images were captured after averaging 64 times, which means that we extracted fluorescent images of cells that did not move for 1 to 2 seconds. From the obtained images, the area covered by adherent platelets was calculated at 5-second intervals as an indicator of platelet adhesion. The extent of platelet adhesion was calculated from the surface area covered by adherent platelets and expressed as the percent area covered by platelets per second as already described. The area coverage of firm adhesion was obtained by subtracting the area coverage of the temporarily arrested platelets7 from that of platelet adhesion. The area coverage thus obtained indicates the area covered by platelets adhering to the same place for at least 5 seconds. The extent of firm platelet adhesion was also expressed as the percent area covered by platelets per second.
Platelet adhesion was also measured by the “fixing adhesion assay.”7,18,19 Glass cover slips were coated with type III bovine collagen by spraying it as a solution from an air brush, which gave a density of 300 ng protein/mm2 of the glass. The cover slips were also coated with vWF by air brush spraying, giving a density of 30 ng protein/mm2 of the glass. Alternatively, the cover slips were coated with vWF or collagen by incubating them with vWF solution (50 μg/mL) or collagen solution (100 μg/mL) overnight in the cold.
Two coated cover slips, one collagen-coated and the other vWF-coated, were individually inserted into the separate receptacles of a flat-plate perfusion chamber. Thus, the vWF- and collagen-coated surfaces would be subjected to the same conditions of blood flow. Whole blood was circulated through the perfusion chamber at a shear rate of 800 s−1 for 2, 5, and 10 minutes. The cover slips were rinsed with phosphate buffer, fixed with 0.5% glutaraldehyde solution, and stained with 0.02% toluidine blue solution. Platelet adhesion was monitored by a light microscope, and the extent of adhesion was expressed as a percentage of the total surface of the cover slip screened.
Measurement of platelet movement. The movement of platelets after they became attached to the vWF- or collagen-coated surface was measured as follows. Fluorescent images were taken every 0.33 to 2 seconds, depending on the velocity as determined from the videotape with an averaging of four frames. The positions of single platelets that moved within the observation period were recorded, and the velocities of each single movement of the platelets were calculated and expressed as micrometers per second. The speeds of about 100 platelet movements on the vWF surface and those of about 30 platelet movements on the collagen surface were used to calculate average velocity on the respective surface.
Effect of reagents on platelet adhesion. Adhesion of platelets to the vWF-coated surface was measured in the presence of platelet aggregation inhibitors at room temperature. To the mepacrine-loaded blood, 5 mmol/L EDTA or 1 mmol/L GRGDS (final concentrations) were added 5 to 30 minutes before the adhesion assay, or anti-GP IIb/IIIa antibody, P2, was added 30 to 60 minutes before the assay at a final concentration of 10 μg/mL. Platelet adhesion was monitored as already described.
Two aggregation inducers, ristocetin and botrocetin, were tested for their effects on platelet adhesion to the vWF-coated surface. Ristocetin (Chrono-Log Corp, Havertown, PA) was added to mepacrine-loaded blood, and platelet adhesion was measured. Botrocetin was tested by first incubating the vWF-coated glass with a 3-μg/mL solution of this inducer for 30 to 60 minutes, washing away the botrocetin solution with HEPES-Tyrode containing 2% BSA, and then flowing the mepacrine-loaded blood over this botrocetin-pretreated, vWF-coated surface.
Statistical analyses. The statistical significance of the difference between each data set was evaluated by the unpaired Student's t-test with the software Prism (Graph Pad Software, San Diego, CA).
RESULTS
Adsorption of the adhesive proteins to various types of glass surfaces. We tested three different glass surfaces for the ability to adsorb adhesive proteins: (1) glass not treated with any reagents (“native glass”), (2) siliconized glass prepared by treating the glass slide with (aminopropyl)triethoxysilane, and (3) ethanol-washed glass. vWF and collagen were labeled with 125I and incubated on each type of glass surface under the same conditions used to prepare unlabeled collagen-coated glass slides for the adhesion assays. The amount of each protein adsorbed on a glass slide was calculated from radioactivity left on the glass after washing and expressed as nanograms of protein per square millimeter.
The three types of glass slides adsorbed similar amounts of vWF (Fig 1A). However, more collagen was adsorbed on the siliconized glass (Fig 1B), and the amount of adhesion on this type of coated surface was much less than that on the vWF-coated native glass slide. Native glass slides were used to measure platelet adhesion onto vWF, inasmuch as vWF adsorption was not affected by the type of glass surface. Since we could not see any difference between platelet aggregation on the collagen adsorbed onto the native glass versus the siliconized glass, siliconized glass slides were used in the experiments measuring platelet adhesion because siliconized glass slides adsorbed more collagen than native glass slides, thus enabling us to prepare a collagen surface with a density similar to that of the vWF surface.
Relationship between the protein amount adhering to the glass and the protein concentration of the incubation solution. 125I-vWF (A) and 125I-collagen (B) solutions of various concentrations were incubated with native glass (▪), ethanol-washed glass (▵), and siliconized glass (○) slides, and the amount of the adsorbed protein on the glass surfaces was calculated from the radioactivity.
Relationship between the protein amount adhering to the glass and the protein concentration of the incubation solution. 125I-vWF (A) and 125I-collagen (B) solutions of various concentrations were incubated with native glass (▪), ethanol-washed glass (▵), and siliconized glass (○) slides, and the amount of the adsorbed protein on the glass surfaces was calculated from the radioactivity.
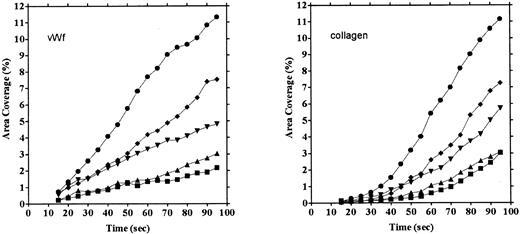
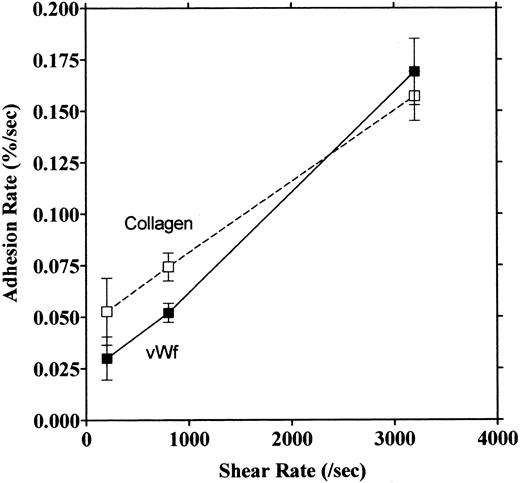
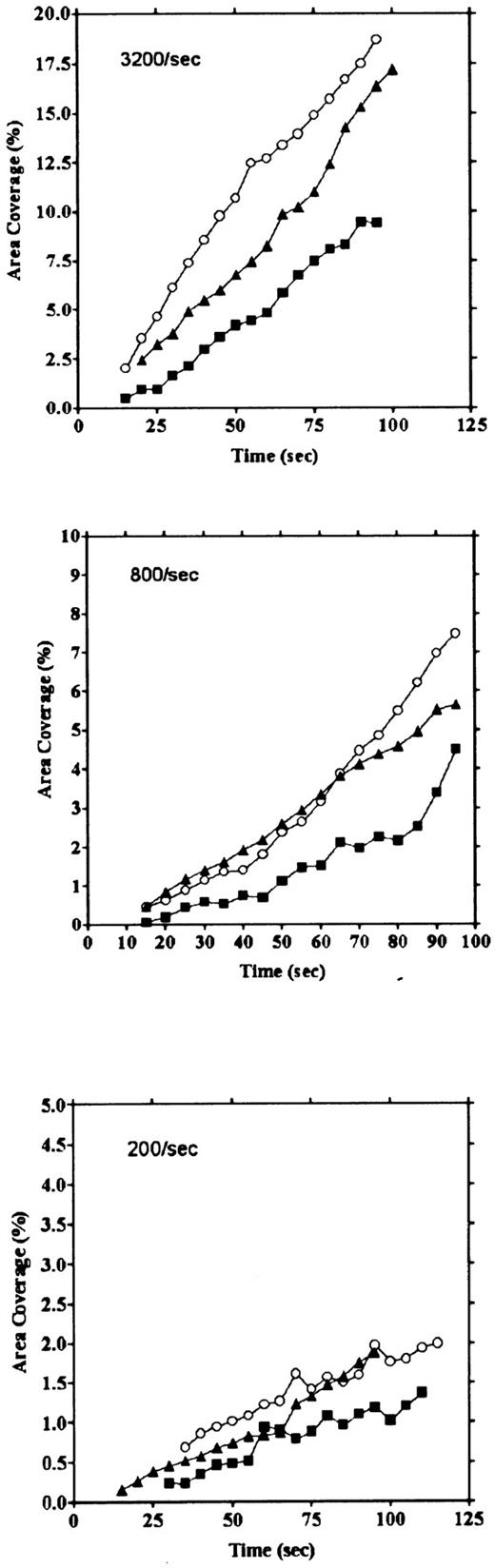
Platelet adhesion on vWF surface. Platelet adhesion on the vWF-coated surface was measured under several shear rates. The pattern of adhesion on vWF was different from that on the collagen surface (Fig 2). For the most part, platelets adhered as individual cells; there were also some aggregates present, but these were notably much smaller in size and formed at a later time than those observed on the collagen-coated surface. The extent of aggregate formation showed some variation among the different blood samples. Adhesion onto the vWF-coated surface, expressed as the percent of area coverage, increased linearly with time, as contrasted with adhesion onto the collagen-coated surface, which was characterized by a biphasic increase (Fig 3). Figure 4 summarizes adhesion rates calculated for the vWF- and collagen-coated surfaces under three different shear rates. Although the adhesion rate to both surfaces was not different under the shear rate of 3,200/s, platelets adhered more rapidly to the collagen-coated surface than to the vWF-coated surface at shear rates of 200/s and 800/s. This phenomenon suggests that platelet aggregation contributes to the adhesion process, especially under low shear. Furthermore, adhesion is linearly correlated with the shear rate for both the vWF- and collagen-coated surfaces (Fig 4). Because the shear rate is closely related to the flow rate, these results suggest that adhesion of platelets to collagen and vWF surfaces mainly depends on the supply of platelets to the surfaces.
Fluorescent images of platelets adhering to vWF-coated and collagen-coated surfaces. Mepacrine-treated blood was passed through the flow chamber at the shear rate of 800/s. Fluorescent patterns were captured at 0.5, 1, and 2 minutes after the start of flow. On the vWF-coated surface, platelets adhered mainly as single platelets, but some small aggregates were observed (arrows). Bars shown in the upper left corner in the top row indicate a length of 50 μm.
Fluorescent images of platelets adhering to vWF-coated and collagen-coated surfaces. Mepacrine-treated blood was passed through the flow chamber at the shear rate of 800/s. Fluorescent patterns were captured at 0.5, 1, and 2 minutes after the start of flow. On the vWF-coated surface, platelets adhered mainly as single platelets, but some small aggregates were observed (arrows). Bars shown in the upper left corner in the top row indicate a length of 50 μm.
Comparison of time courses of area coverage of platelets adhering to vWF- and collagen-coated surfaces. Values for the percentage of area covered by fluorescent platelets were plotted as a function of the time after the start of blood flow. Adhesion was observed under flow rates of 200/s (▪), 400/s (▴), 800/s (▾), 1,600/s (♦), and 3,200/s (•). Adhesion on the collagen-coated surface increased biphasically, but adhesion on the vWF-coated surface increased linearly.
Comparison of time courses of area coverage of platelets adhering to vWF- and collagen-coated surfaces. Values for the percentage of area covered by fluorescent platelets were plotted as a function of the time after the start of blood flow. Adhesion was observed under flow rates of 200/s (▪), 400/s (▴), 800/s (▾), 1,600/s (♦), and 3,200/s (•). Adhesion on the collagen-coated surface increased biphasically, but adhesion on the vWF-coated surface increased linearly.
Relationship between adhesion rate and shear rate. Rates of platelet adhesion on collagen (□) and on vWF (▪) were calculated and expressed as a function of different shear rates. The second phase of collagen adhesion was used for calculation of the adhesion rate. Mean values were calculated from 9 to 13 experiments, and the bars indicate the SEM.
Relationship between adhesion rate and shear rate. Rates of platelet adhesion on collagen (□) and on vWF (▪) were calculated and expressed as a function of different shear rates. The second phase of collagen adhesion was used for calculation of the adhesion rate. Mean values were calculated from 9 to 13 experiments, and the bars indicate the SEM.
Platelet adhesion to the vWF-coated surface was also monitored by a different method, the fixing adhesion assay in which adherent platelets were observed under a microscope after fixing and staining. The vWF- and collagen-coated glass plates were placed in series so that blood flow conditions over both plates would be the same. Figure 5 shows the staining pattern of platelets adhered to the vWF- and collagen-coated surfaces. The morphologic pattern of platelet adhesion on the vWF surface was different from that on the collagen surface. With the fixing adhesion assay, the pattern differences were similar to those illustrated in Fig 2 but much more pronounced. On the vWF surface, platelets adhered separately as single cells and also formed small aggregates, markedly different from the large aggregates formed on the collagen surface. When adhesion was quantified as area coverage, the percentage of the area covered by platelets was not significantly different between the two types of adhesion (Table 1). In these experiments, vWF and collagen were coated on the glass surface by spraying and adsorption; the spraying method gave vWF and collagen densities of 30 and 300 ng/mm2, respectively, and the adsorption method gave densities of 4 and 3 ng/mm2, respectively. Despite the 10- and 100-fold difference in coated protein density for vWF and collagen, respectively, between the two types of coating methods, the surfaces showed similar area coverage values (Table 1).
Comparison of the morphology of platelets adhering to the collagen-coated surface and the vWF-coated surface (fixing adhesion method). Normal blood was circulated through the perfusion chamber for 5 minutes under a shear rate of 800/s, and adherent platelets were fixed, stained, and then observed by microscopy. The formation of large platelet aggregates was observed on the collagen-coated surface.
Comparison of the morphology of platelets adhering to the collagen-coated surface and the vWF-coated surface (fixing adhesion method). Normal blood was circulated through the perfusion chamber for 5 minutes under a shear rate of 800/s, and adherent platelets were fixed, stained, and then observed by microscopy. The formation of large platelet aggregates was observed on the collagen-coated surface.
vWF dependency of the adhesion to the collagen-coated surface. Platelet adhesion to the collagen-coated surface was measured in the presence of the anti-GP Ib monoclonal antibody NNKY5-5 or the anti-vWF monoclonal antibody AJvW-2. Both monoclonal antibodies strongly inhibited platelet adhesion to the collagen surface (data not shown). Especially striking is the lack of temporary arrest on the collagen surface in these experiments. These results suggest that vWF and GP Ib on the platelet surface contribute to the early interaction between collagen and platelets, the temporary arrest.
To confirm this hypothesis, collagen-coated glass slides were incubated with 50 μg/mL vWF solution and washed in HEPES-Tyrode solution, and then platelet adhesion on these glass slides was monitored. The vWF-incubated, collagen-coated surface showed an adhesion curve similar to that of the vWF surface (Fig 6). The collagen-coated glass incubated with plasma also gave similar results (data not shown).
Effect of preincubation of vWF solution with the collagen-coated surface. Collagen-coated glass slides were preincubated with vWF solution (50 μg/mL) at 4°C overnight, and adhesion was measured under the shear rates of 200, 800, and 3,200/s (○). As controls, platelet adhesion on collagen- (▪) and vWF-coated (▴) surfaces was also measured.
Effect of preincubation of vWF solution with the collagen-coated surface. Collagen-coated glass slides were preincubated with vWF solution (50 μg/mL) at 4°C overnight, and adhesion was measured under the shear rates of 200, 800, and 3,200/s (○). As controls, platelet adhesion on collagen- (▪) and vWF-coated (▴) surfaces was also measured.
Taken together, these observations suggest that the binding of vWF in blood to the collagen-coated surface would be the first reaction in platelet adhesion to the collagen surface, and the temporary arrest would imply the interaction of platelets with vWF bound to the collagen surface.
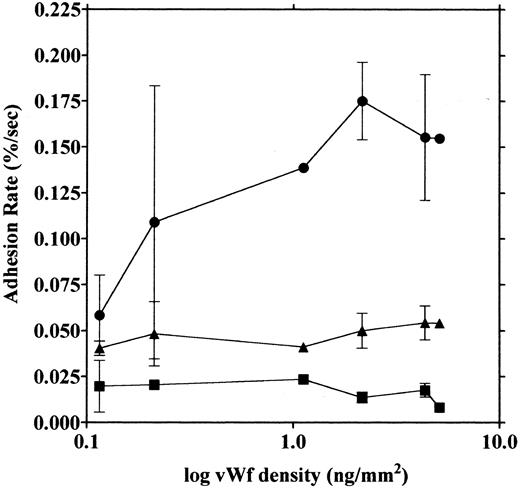
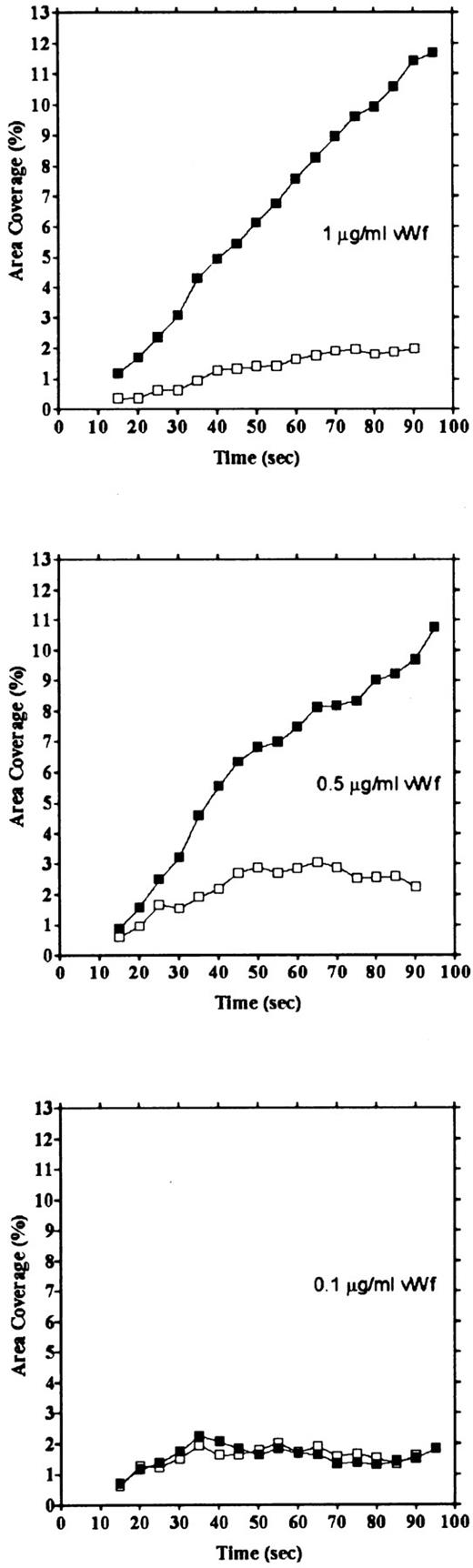
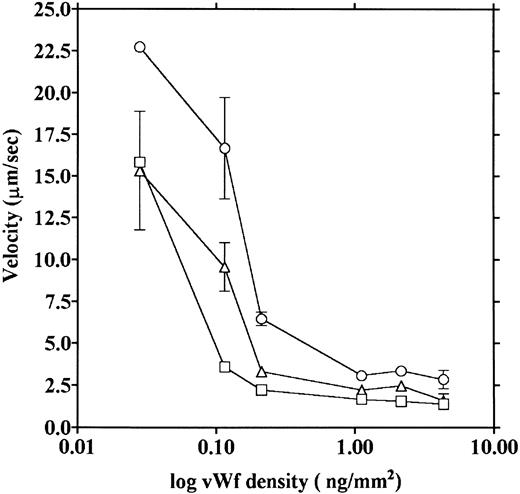
vWF concentration dependency of platelet adhesion. Platelet adhesion was measured using glass cover slips adsorbed with vWF solutions of different concentrations under three different shear rates. Figure 7 shows the relationship between the adhesion rate and vWF density on the glass surface calculated from the relationship shown in Fig 1. In the range of 0.12 to 5.1 ng/mm2 vWF on the surface, platelet adhesion at shear rates of 200/s and 800/s was similar; however, under the shear rate of 3,200/s, platelet adhesion at the lowest density of coated vWF was decreased to the 800/s level. At the lowest density of coated vWF (0.028 ng/mm2 ), adhesion was very low, most of it being temporary arrest (Fig 8). As can be seen by Fig 8 showing platelet adhesion and temporary arrest under the shear rate of 3,200/s at three densities of coated vWF, the contribution of temporary arrest to platelet adhesion increases when vWF density on the surface decreases. Under the lower shear rates, similar tendencies were observed, although the effects were small (data not shown). Higher vWF densities facilitate adhesion, especially under high shear rates, while at lower vWF densities, temporary arrest becomes the predominant interaction because the limited number of vWF molecules would be insufficient to hold on to the platelets. The velocities of moving platelets were also measured for these experiments. Velocities of platelets moving on the vWF-coated surface increased as the density of coated vWF became lower than about 0.2 ng/mm2 (Fig 9). These results indicate that platelets move faster and adhere less on the vWF-coated surface with low density, suggesting that adhesion of platelets to the vWF-coated surface mainly depends on the level of interaction with the coated vWF molecules.
Platelet adhesion on surfaces coated with vWF at different densities. Glass slides were coated with different concentrations of vWF, and densities of vWF adsorbed on the glass surfaces were calculated from the relationship shown in Fig 1. Platelet adhesion was measured under shear rates of 200 (▪), 800 (▴), and 3,200/s (•). The mean ± SEM of 2 series of experiments were plotted, except for the values without bars that indicate values from single experiments and the values of 4.35 ng/mm2 that represent the average of five experiments.
Platelet adhesion on surfaces coated with vWF at different densities. Glass slides were coated with different concentrations of vWF, and densities of vWF adsorbed on the glass surfaces were calculated from the relationship shown in Fig 1. Platelet adhesion was measured under shear rates of 200 (▪), 800 (▴), and 3,200/s (•). The mean ± SEM of 2 series of experiments were plotted, except for the values without bars that indicate values from single experiments and the values of 4.35 ng/mm2 that represent the average of five experiments.
Platelet adhesion and temporary arrest on different densities of vWF. Platelet adhesion was measured on surfaces with different densities of vWF. Glass slides were incubated with 1, 0.5, and 0.1 μg/mL vWF solution, for densities of 0.212, 0.115, and 0.028 ng/mm2, respectively. Adhesion under the shear rate of 3,200/s was calculated as area coverage (▪) and temporary arrest (□).
Platelet adhesion and temporary arrest on different densities of vWF. Platelet adhesion was measured on surfaces with different densities of vWF. Glass slides were incubated with 1, 0.5, and 0.1 μg/mL vWF solution, for densities of 0.212, 0.115, and 0.028 ng/mm2, respectively. Adhesion under the shear rate of 3,200/s was calculated as area coverage (▪) and temporary arrest (□).
Platelet movement on vWF surfaces of different densities. Velocities of platelet movement in the same experiments as shown in Fig 7 were measured under shear rates of 200 (□), 800 (▵), and 3,200/s (○), and the velocities were plotted as a function of vWF density on the glass.
Platelet movement on vWF surfaces of different densities. Velocities of platelet movement in the same experiments as shown in Fig 7 were measured under shear rates of 200 (□), 800 (▵), and 3,200/s (○), and the velocities were plotted as a function of vWF density on the glass.
From measurements of moving platelets on the collagen surface, the calculated velocities were 26.58 ± 4.37 and 49.76 ± 7.92 μm/s (mean ± SEM, n = 4) for shear rates of 800/s and 3,200/s, respectively; these values are about twice those obtained from adhesion on the lowest density of adsorbed vWF. However, when velocities on the collagen surface were measured after 60 seconds from the start of blood flow, which corresponded to the time of the second phase of the adhesion (Fig 3), the velocities were 12.62 ± 1.17 and 12.83 ± 1.76 μm/s (mean ± SEM, n = 5) for 800/s and 3,200/s, respectively. These values correspond to the values for adhesion to the vWF surface with a density of about 0.1 ng/mm2. In these experiments, most of the platelets remained stationary on the collagen surface, and we only measured platelets that moved during the observation period. These results suggest that vWF in the sample blood adheres to the collagen surface, and the amount of vWF adherent on the collagen surface would increase with flow time.
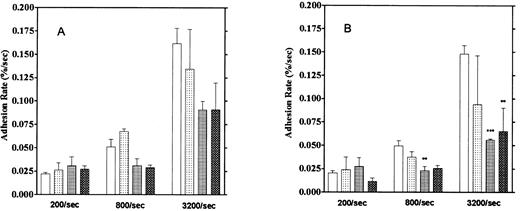
Effect of aggregation inhibitors. Platelet adhesion to the vWF-coated surface was measured in the presence of aggregation inhibitors, 5 mmol/L EDTA, 1 mmol/L GRGDS peptide, and the anti-GP IIb/IIIa monoclonal antibody P2 (10 μg/mL). Here, we calculated adhesion by two methods. One method was the usual one that observes platelets adhering for 1 to 2 seconds (“short adhesion”). In the second method, platelet adhesion was calculated from images that did not move for more than 5 seconds, which we defined as “firm adhesion”. These reagents showed stronger inhibitory activities on firm adhesion, especially at high shear rates, than on short adhesion (Fig 10). Inhibition of firm adhesion by the monoclonal antibody P2 was especially pronounced at shear rates of 800/s and 3,200/s. We also observed that more platelets were moving on the vWF surface in the presence of these inhibitors than in their absence. Furthermore, the formation of small platelet aggregates usually observed under higher shear rates was completely blocked by these inhibitors. These results suggest the contribution of GP IIb/IIIa to the firm adhesion of platelets on the vWF surface, especially under high shear rates.
Effect of aggregation inhibitors on platelet adhesion on the vWF-coated surface. (A) Platelet adhesion with temporary arrest. (B) Platelet adhesion without temporary arrest. Platelet adhesion was measured in the presence of 1 mmol/L GRGDS (▧), 10 μg/mL anti-GP IIb/IIIa antibody (▦), or 5 mmol/L EDTA (▩) under the 3 different shear rates. (□) Control data. The mean ± SEM are results from five different experiments for anti-GP IIb/IIIa, 10 for the control, 3 for GRGDS, and 3 for EDTA.
Effect of aggregation inhibitors on platelet adhesion on the vWF-coated surface. (A) Platelet adhesion with temporary arrest. (B) Platelet adhesion without temporary arrest. Platelet adhesion was measured in the presence of 1 mmol/L GRGDS (▧), 10 μg/mL anti-GP IIb/IIIa antibody (▦), or 5 mmol/L EDTA (▩) under the 3 different shear rates. (□) Control data. The mean ± SEM are results from five different experiments for anti-GP IIb/IIIa, 10 for the control, 3 for GRGDS, and 3 for EDTA.
Effects of botrocetin and ristocetin. Mepacrine-loaded blood was mixed with ristocetin (0.6 mg/mL) and passed through the perfusion chamber. Adhesion rates were not different from those in the control experiments; however, the formation of loose platelet aggregates and advanced platelet adhesion was observed on the surface near the slit for blood entrance (data not shown). Next, we incubated the vWF surface with botrocetin solution (3 μg/mL), and the adhesion assay was performed after washing out the botrocetin solution with HEPES/Tyrodes with 2% BSA, so that platelets in the blood reacted with the botrocetin-vWF complex on the surface. Botrocetin slightly reduced platelet adhesion to vWF, especially under higher shear rates. However, platelets were observed to adhere very densely near the inlet of blood flow and very scarcely near the outlet of blood flow. Also, formation of larger aggregates was observed. These results suggest that the botrocetin-vWF surface is very adhesive and can thereby tightly arrest many platelets, so that platelets accumulate on the surface near the inlet of blood flow and much fewer platelets flow to the downstream portion of the chamber; this would explain why botrocetin reduced platelet adhesion at the middle of the flow path where the measurements were usually performed.
We observed that platelets attached to the botrocetin-vWF surface essentially did not move. Platelets moved only once or twice just after contact to the botrocetin-vWF surface, which is different from adhesion to the vWF surface, where most platelets moved continually. The velocities of platelets moving on the vWF-coated surface in the presence of ristocetin or botrocetin were measured. Figure 11 shows that velocities over the vWF-botrocetin surface and those over the vWF surface in the presence of ristocetin significantly decreased. Also, 5 mmol/L EDTA had no effect on the velocities over the vWF-botrocetin surface (data not shown). These results indicate that botrocetin and ristocetin increase the affinity of the interaction between platelets and vWF.
Effect of botrocetin and ristocetin on the velocities of platelet movements. Velocities of platelets moving on the vWF-coated surface in the presence of ristocetin (0.6 mg/mL) and those of platelets moving on the botrocetin-treated vWF surface were measured. Values are the average of three experiments, except for the controls, which are from nine experiments.
Effect of botrocetin and ristocetin on the velocities of platelet movements. Velocities of platelets moving on the vWF-coated surface in the presence of ristocetin (0.6 mg/mL) and those of platelets moving on the botrocetin-treated vWF surface were measured. Values are the average of three experiments, except for the controls, which are from nine experiments.
DISCUSSION
The essential contribution of vWF to platelet adhesion to the vessel wall, especially under high shear rates, has been well established. vWF was also shown to contribute to platelet adhesion to collagen-,20,21 fibrin-,22 and fibronectin-coated23 surfaces under flow conditions, and the A3 domain of vWF has been identified as the collagen binding domain.24 vWF was suggested to bind to these adhesive protein-coated surfaces first, and then the immobilized vWF would bind to GP Ib on the platelets, resulting in a localization of the platelets on the adhesive surface. However, platelet activation does not occur under normal physiologic conditions even though there is a sufficient level of vWF in the blood; and furthermore, the binding of platelets with vWF molecules in solution could not be detected by conventional binding assays. Because platelets bind to immobilized vWF under the static condition,4,25 immobilization was suggested to expose the platelet binding site of vWF. Recently, another hypothesis was presented by Ruggeri's group, who found a reversible binding of vWF5 to platelets in solution and also observed that platelets moved on the vWF-coated surface under flow conditions.6 They suggested that the reversible binding of vWF to platelets is derived from the high association rate and the high dissociation rate, and this reversible binding and shear stress induce activation of GP IIb/IIIa, resulting in the tight binding of platelets and vWF and platelet aggregation.5 6
The data obtained in the present experiments support previously reported results.5,6 Platelets were observed to move on the vWF-coated surface. We also demonstrated that platelet adhesion and platelet moving velocity were both dependent on the concentration of vWF on the glass surface. When the concentration of vWF on the glass surface was decreased, platelet adhesion decreased, especially under a high shear rate (Fig 7), and the velocity of platelet movement increased (Fig 9). Platelet adhesion and movement plateaued at the higher densities of coated vWF; at the lower densities of coated vWF, adhesion was characterized by a higher percentage of temporary arrest (Fig 8). These results suggest that the interaction between vWF and platelets is a weak one, and the velocity of platelet movement and platelet adhesion depend on the number of these interactions, although this concentration dependency was observed only at the low concentration of vWF. It is notable that the moving velocities reached their lower limit at the high densities of coated vWF; this suggests that platelets would not stop even when they make a maximum number of linkages with the vWF surface. Our measured velocities of platelet movement were in a range similar to the values reported by Savage et al.6
As to the effect of platelet aggregation inhibitors on platelet adhesion to the vWF surface, Wu et al20 showed that an anti-GP IIb/IIIa monoclonal antibody and PGI2 strongly inhibited this interaction. Savage et al6 indicated that the adhesion of nondetachable platelets was inhibited by PGE1 and another anti-GP IIb/IIIa monoclonal antibody; the results illustrated in Fig 10 are similar to theirs. We found that the aggregation antagonists did not significantly inhibit short adhesion, ie, adhesion where platelets stayed for 1 to 2 seconds at the same place, in contrast to their inhibitory effect on firm adhesion. Wu et al20 performed their experiments with the fixing adhesion assay, so the adhesion they observed after washing and fixation would also be platelets that adhered firmly onto the vWF surface. These results suggest the contribution of GP IIb/IIIa to the firm adhesion of platelets on vWF under flow conditions. The present observations indicate that these inhibitory effects of aggregation inhibitors on platelet adhesion on the vWF surface are shear rate–dependent. Under a lower shear rate, platelet adhesion was not affected by these reagents. The previous reports6,20 provided data obtained at high shear rates (about 1,500/s), but provided no low–shear rate results. These inhibitors strongly inhibited platelet adhesion on the collagen-coated surface even under low shear rates,7 so the shear rate dependency of aggregation inhibitors would be unique to adhesion on the vWF surface. Since platelet surface GP IIb/IIIa was shown to be activated by shear stress,26 these GPs would be involved in the firm adhesion process. Savage et al25 suggested that the binding of vWF and GP Ib would induce a change in GP IIb/IIIa that would enable the complex to bind to bound vWF.
This study is the first to analyze the effects of ristocetin and botrocetin on platelet adhesion on the vWF surface under flow conditions. Botrocetin and ristocetin are known to bind to vWF, increase the affinity of vWF for GP Ib, and induce platelet aggregation. Binding sites for these reagents were identified inside the loop of the A1 domain in the vWF molecule.3,27,28 We found that botrocetin and ristocetin reduced platelet movement on the vWF surface (Fig 11). Most of the adherent platelets did not move on the botrocetin-treated vWF surface; they moved only once or twice after their first contact on the surface and thereafter remained stationary. These results indicate that the affinity of vWF for platelets was increased by botrocetin. In the presence of ristocetin, platelets showed only slightly more movement. According to the reversible binding hypothesis,5,6 this suggests that botrocetin changes the dissociation rate of the vWF-GP Ib interaction. The dissociation rate would decrease while the association rate would remain high, resulting in a lower dissociation constant. Previously reported binding experiments of vWF molecules to platelets also indicated that there was increased binding in the presence of either ristocetin or botrocetin.29 30 In those studies, no binding of vWF to platelets could be observed in the absence of these reagents. Thus, these reagents would be expected to markedly decrease the dissociation rate of vWF from platelets, although the mechanism for this action remains unknown. Since GP IIb/IIIa contributes to the firm adhesion of platelets to the vWF surface, the involvement of these GPs in the firm adhesion induced by botrocetin and ristocetin is another possible hypothesis.
A main purpose of this report is to compare platelet adhesion on the collagen-coated surface with that on the vWF-coated surface under flow conditions. Although platelet adhesion on these surfaces was demonstrated by a number of investigators,6-8,20,21 31 few of them described how adhesion on the two surfaces differs. In the present study, we were able to demonstrate three differences in adhesion to these two surfaces by two different assay procedures. The most striking difference is that platelets adhere to the vWF surface mainly as a single cell, in contrast to adhesion on the collagen surface, where platelets adhered mainly as aggregates (Figs 2 and 5). In Fig 5 (fixing adhesion assay), platelets formed much larger aggregates on the collagen surface as compared with those in Fig 2 (fluorescent assay). This is because the flow time in the fixing adhesion assay was longer, allowing the platelet aggregates to grow larger, and also because the higher density of absorbed collagen in this assay supported greater aggregate formation. We observed that the platelets adhere as single cells during the early flowing time, and later, multiplatelet aggregates are formed, which grow larger with time; therefore, in the later stage, only large aggregates were observed.
Another difference of platelet adhesion on these two surfaces is the pattern of the adhesion increase. When adhesion was measured by the percent area occupied by adherent platelets, adhesion on collagen surfaces usually increased biphasically with flowing time, while adhesion on vWF surfaces usually increased linearly (Fig 3). The biphasic increase of platelet adhesion on the collagen surface was changed to a monophasic increase by incubating the collagen surface with vWF solution before initiating blood flow (Fig 6). Our present data, together with previous studies showing a strong inhibition of adhesion on collagen by monoclonal antibodies against vWF and GP Ib,20 21 are consistent with the following scheme: vWF binds to collagen first, and this bound vWF would be a prerequisite for adhesion of platelets on the collagen surface under flow conditions.
The third difference in adhesion to collagen and vWF surfaces is how the platelets move on these two surfaces. We observed that many platelets moved on the vWF surface, and their movements were more constant and slower than those on the collagen surface. We did not measure platelet movement in the presence of PGE1 as reported by Savage et al,6 so we did not observe that all platelets were moving on the vWF surface, but a considerable percentage of the platelets were moving and the velocities of the moving platelets were consistent with their results. On the other hand, most of the platelets remained at the same spot on the collagen surface, and if the platelets moved, their motions were irregular and faster than those on the vWF surface.7 The velocities of moving platelets on the collagen surface measured from 25 seconds after initiation of blood flow were two times higher than the values obtained for the vWF surface of the lowest density (∼0.028 ng/mm2 ). However, the velocities on the collagen surface decreased to the level of those on the vWF surface with the density of 0.1 ng/mm2 when the velocities were measured at about 1 minute after initiation of flow. These results suggest that the amount of vWF adsorbed on the collagen surface increases with time while the blood is flowing if the moving velocity of platelets is only dependent on vWF on the surface. The presence of biphasic adhesion on the surface would be explained by the time of vWF binding to the collagen surface. At the early time points, the interaction of platelets with the surface would be temporary because the density of vWF on the surface is very low, and the platelets in the flow would be more strongly attracted to the vWF-collagen surface after the density of adsorbed vWF on the surface increases to a certain level.
We do not know what density of vWF on the collagen surface would be sufficient to induce second-phase adhesion. However, Roth et al32 measured the binding of vWF to immobilized collagen and estimated the molar ratio of bound vWF to immobilized collagen to be 1 to 15. Because collagen on the glass surface can be estimated to be 1 to 2 ng/mm2 from the relationship shown in Fig 1, the density of vWF on the collagen surface can be estimated to be 0.07 to 0.15 ng/mm2. These densities of vWF would result in increased platelet moving velocities in accordance with the relationship shown in Fig 9, and the values are similar to those estimated from the velocities on the collagen surface at 60 seconds after initiation of flow. From these results, we can estimate that the density of vWF on the collagen surface is low, about 0.1 ng/mm2, even in the second phase of adhesion; under this condition, the platelets still moved faster on the vWF-collagen surface than in the usual condition of platelet adhesion on the vWF surface where the density of vWF is much higher.
We can make the following conclusions that are consistent with the present data and our previous observations on platelet-collagen interaction under flow conditions. Because the concentration of vWF bound to the collagen surface is low and the velocities of platelets moving on the collagen surface are fast, immobilized vWF on the collagen surface would weakly interact with the platelets, slowing them and attracting them to come closer to the collagen surface. vWF would not have a major function in the next reaction, the firm arrest of platelets on the collagen. Platelets slowed by the interaction with vWF would bind with collagen through collagen receptors on the platelet surface. GP Ia/IIa33,34 and/or GP VI7,35 were reported to be involved in platelet adhesion to collagen. The interaction of collagen receptors and collagen would firmly reinforce the binding of platelets initially facilitated by the vWF interaction, resulting in enhanced platelet adhesion and aggregation. Both GP Ia/IIa and GP VI would be necessary for full activation of platelets under flow conditions, because GP VI–deficient platelets can adhere to the collagen surface but cannot form aggregates.7 How the interaction of collagen with GP VI differs from the collagen–GP Ia/IIa interaction should be analyzed under flow conditions.
ACKNOWLEDGMENT
We thank Drs Takashi Morita and Fujio Sekiya (Meiji College of Pharmacy, Tanashi, Tokyo, Japan) for the kind gift of purified botrocetin; Dr Ryota Yoshimoto (Central Research Lab, Ajinomoto Co, Yokohama, Japan) for providing the monoclonal antibody AJvW-2; and Drs Michio Matsuda (Jichi Medical School, Tochigi, Japan) and Gilbu Soe (Central Research Lab, Iatron Laboratories Inc, Chiba, Japan) for providing the monoclonal antibody IF-1. We also thank Ryoko Taguchi for technical assistance.
Supported by a grant (07833012) from the Ministry of Education, Science, Sports, and Culture of Japan (M.M.), grants (DGECYT PM95/0103 and CIRIT GR093-9121) to A.O., and an Invitation Fellowship from the Japan Society for the Promotion of Science (M.D.R.).
Address reprint requests to Masaaki Moroi, PhD, Department of Protein Biochemistry, Institute of Life Science, Kurume University, 2432-3 Aikawa-machi, Kurume, Fukuoka 839, Japan.