Abstract
Members of the hematopoietically expressed Tec tyrosine kinase family have an important role in hematopoietic signal transduction, as exemplified by the crucial role of Btk for B-cell differentiation and activation. Although a variety of cell surface receptors have been found to activate Tec tyrosine kinases, the specific signaling pathways and substrate molecules used by Tec kinases are still largely unknown. In this study a Tec family kinase, Bmx, was found to induce activation of the Stat signaling pathway. Bmx induced the tyrosine phosphorylation and DNA binding activity of all the Stat factors tested, including Stat1, Stat3, and Stat5, both in mammalian and insect cells. Bmx also induced transcriptional activation of Stat1- and Stat5-dependent reporter genes. Other cytoplasmic tyrosine kinases, Syk, Fyn, and c-Src, showed no or only weak ability to activate Stat proteins. Expression of Bmx in mammalian cells was found to induce activation of endogenous Stat proteins without activation of endogenous Jak kinases. We further analyzed the Bmx-mediated activation of Stat1, which was found to be regulated by protein kinase C δ (PKCδ) isoform, but not β 1, ε, or ζ isoforms, leading to inhibition of Stat1 tyrosine phosphorylation. In conclusion, these studies show that Bmx, a Tec family kinase, can function as an activator of the Stat signaling pathway and identify a role for PKCδ in the regulation of Bmx signaling.
PROTEIN TYROSINE phosphorylation is a central signaling mechanism for the growth and differentiation responses in hematopoietic cells. The majority of hematopoietic cell surface receptors, including cytokine and antigen receptors, lack the ability to phosphorylate protein substrates directly. Instead, ligand-induced receptor activation is coupled to downstream signaling events through receptor-associated cytoplasmic protein tyrosine kinases.1
A group of hematopoietically expressed cytoplasmic tyrosine kinases, termed the Tec tyrosine kinase family, has an important role in hematopoietic signal transduction and hematopoietic cell development.2 This family consists of the founder member Tec, Btk, Itk/Tsk/Emt, Txk, and Bmx tyrosine kinases, which share a characteristic domain structure including an amino-terminal pleckstrin homology (PH) domain, Tec homology (TH), src homology 3 (SH3) and 2 (SH2) domains, and a carboxy-terminal catalytic tyrosine kinase domain. Each Tec family kinase has its specific expression pattern. Expression of Btk is restricted to B-lymphoid and myeloid lineages, Itk is expressed predominantly in cells of T-lymphoid lineage, and Txk is expressed in myeloid and T cells, whereas the Tec tyrosine kinase is more widely expressed in hematopoietic cells.2 The expression of Bmx kinase is restricted to myeloid cells and certain endothelial cells.3 4
Thus far the biological functions for two Tec kinases, Btk and Itk, have been revealed and found to be regulating cell type–specific responses, consistent with the restricted expression pattern of these kinases. Naturally occurring mutations in the Btk gene result in human x-linked agammaglobulinemia (XLA) and murine x-linked immunodeficiency (Xid).5,6 The XLA phenotype is characterized by a lack of mature B cells due to developmental defects during pre–B-cell expansion.7,8 Recent studies have also shown that Itk plays a central role in T-cell signaling. Itk knockout mice have reduced numbers of mature thymocytes and show alterations in T-cell antigen receptor signaling.9
Several Tec family tyrosine kinases associate with and are activated through various hematopoietic cell surface receptors. Btk has been found to be activated through the mast cell FcεR, B-cell antigen receptor, interleukin-5 receptor (IL-5R), and the IL-6 cytokine family signal transducer, gp130.10-14 Tec is activated through gp130, the receptor tyrosine kinase c-kit, and the IL-3Rβ chain,14-16 whereas Itk is activated through ligation of CD28 in T cells and FcεR in mast cells.17,18 Thus far no activating receptor has been identified for the Bmx kinase. Activation by multiple cell surface receptors suggests that Tec kinases could directly activate signaling pathways from these receptors. However, the specific target proteins and downstream signaling pathways for Tec kinases and their exact roles in cytokine signaling are still largely unknown. Thus far the only substrate for Tec family kinases is phospholipase γ (PLCγ), which has been found to be regulated by Btk together with Syk following B-cell antigen receptor activation.19
Some of the molecular mechanisms regulating Btk activity have been characterized. Activation of Btk after B-cell receptor crosslinking is preceded by activation of Src family kinases and the Src kinases have been found to be critical regulators of Btk activity. The Src kinase Lyn binds directly through its SH3 domain to the proline-rich region of Btk and phosphorylates tyrosine 551 in the Btk catalytic domain.20-22 This results in enhanced Btk activity and subsequent Btk autophosphorylation at a second site, tyrosine 223 in the SH3 domain.22,23 Btk also associates through its PH domain with βγ subunits of heterotrimeric G proteins and with various isoforms of protein kinase C (PKC).24,25 The interaction with PKC leads to downregulation of Btk activity.25 PKC may be a general regulator for Tec kinases, because Itk has also been found to interact with multiple PKC isoforms.18 However, this interaction results in activation of Itk catalytic activity.18
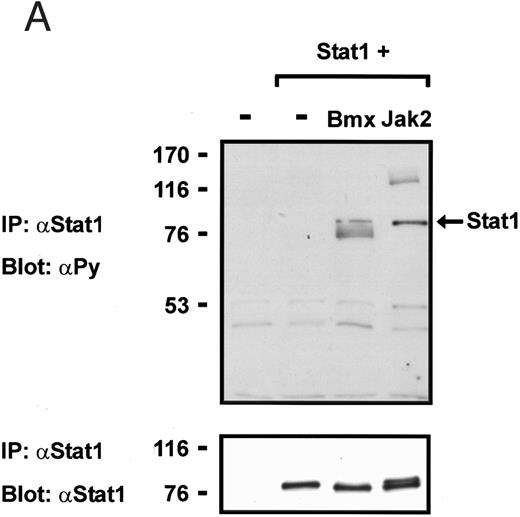
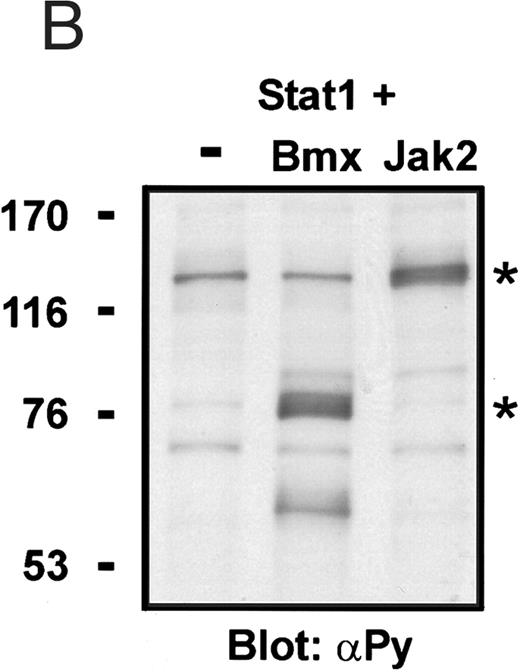
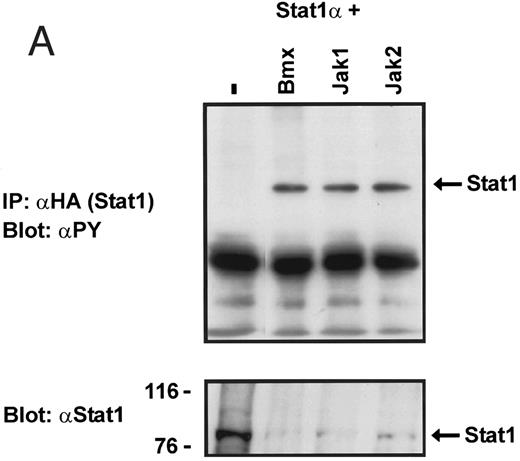
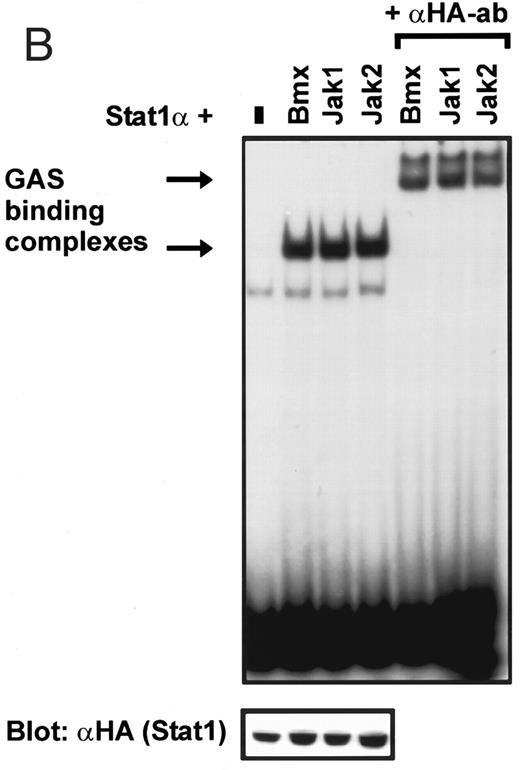
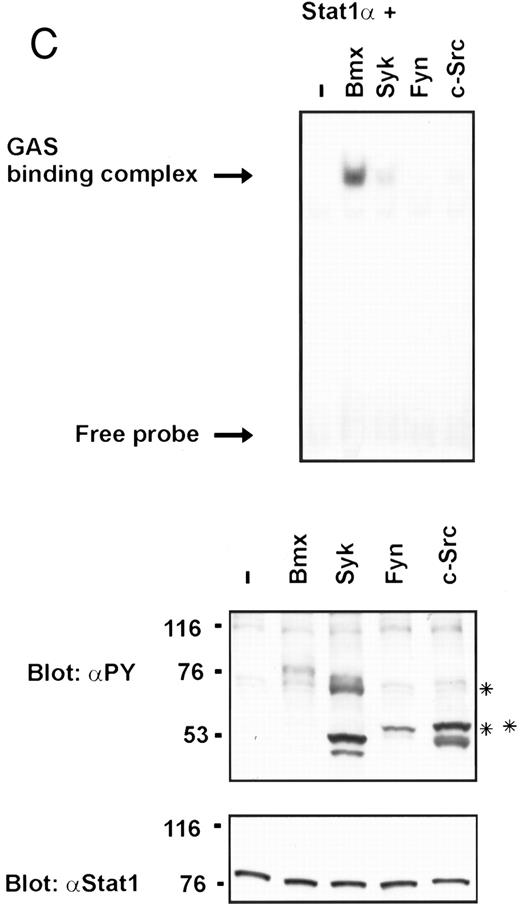
Coexpression of Bmx induces activation of Stat1. (A) COS cells were transfected with Stat1α expression vector together with Bmx, Jak1, Jak2, or control expression vectors, as indicated. Stat1α was immunoprecipitated from cell lysates using anti-HA antibody. The immunoprecipitates were electrophoresed in 7.5% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-phosphotyrosine antibody. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted with anti-Stat1 antibody. (B) COS cells were transfected with Stat1α expression vector together with Bmx, Jak1, Jak2, or control expression vectors, as shown. Eight micrograms of protein from each lysate of transfected cells was incubated with 32P-labeled GAS oligonucleotide and analyzed in the mobility shift assay in 4.5% TBE-PAGE. The Bmx, Jak1, and Jak2 lysates were also supershifted using anti-HA antibody (for Stat1α-HA). Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted for Stat1α using anti-HA antibody. (C) COS cells were transfected with Stat1α expression vector alone or together with Bmx-HA, Syk, Fyn, or c-Src expression vectors. Lysates were analyzed in the mobility shift assay as above. Shown in the lower panels are immunoblots from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted with anti-phosphotyrosine antibody and anti-Stat1 antibody. The autophosphorylated Syk, Fyn, and c-Src polypeptides are indicated by asterisks.
Coexpression of Bmx induces activation of Stat1. (A) COS cells were transfected with Stat1α expression vector together with Bmx, Jak1, Jak2, or control expression vectors, as indicated. Stat1α was immunoprecipitated from cell lysates using anti-HA antibody. The immunoprecipitates were electrophoresed in 7.5% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-phosphotyrosine antibody. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted with anti-Stat1 antibody. (B) COS cells were transfected with Stat1α expression vector together with Bmx, Jak1, Jak2, or control expression vectors, as shown. Eight micrograms of protein from each lysate of transfected cells was incubated with 32P-labeled GAS oligonucleotide and analyzed in the mobility shift assay in 4.5% TBE-PAGE. The Bmx, Jak1, and Jak2 lysates were also supershifted using anti-HA antibody (for Stat1α-HA). Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted for Stat1α using anti-HA antibody. (C) COS cells were transfected with Stat1α expression vector alone or together with Bmx-HA, Syk, Fyn, or c-Src expression vectors. Lysates were analyzed in the mobility shift assay as above. Shown in the lower panels are immunoblots from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted with anti-phosphotyrosine antibody and anti-Stat1 antibody. The autophosphorylated Syk, Fyn, and c-Src polypeptides are indicated by asterisks.
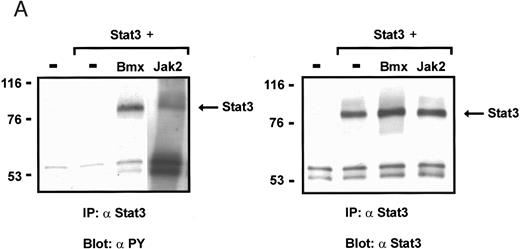
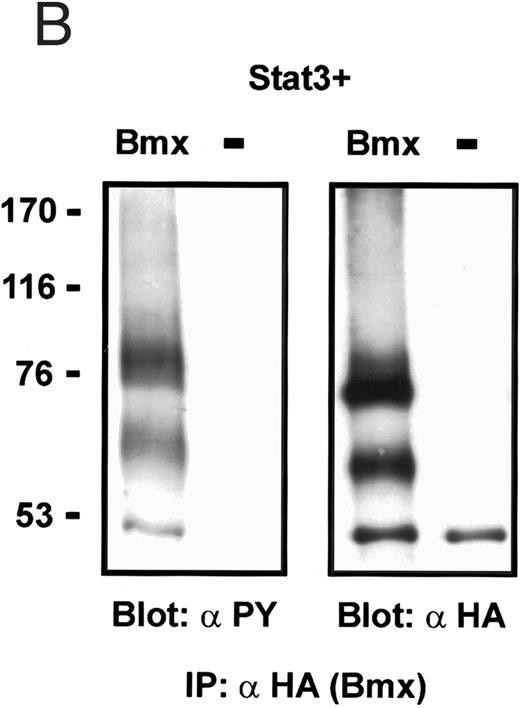
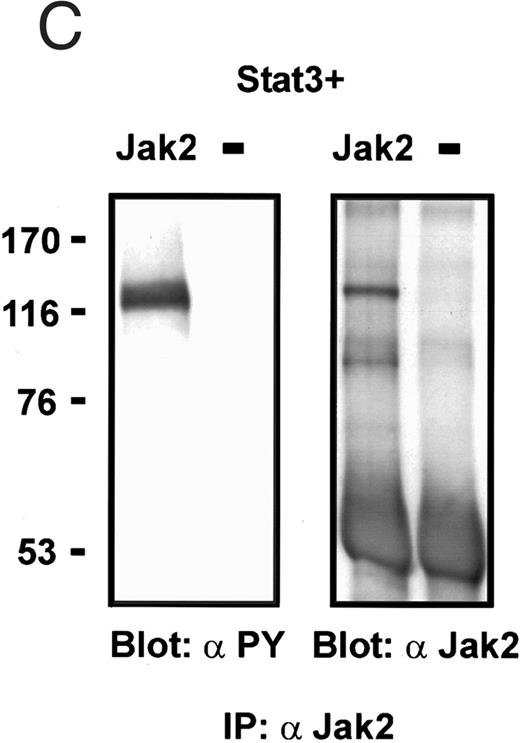
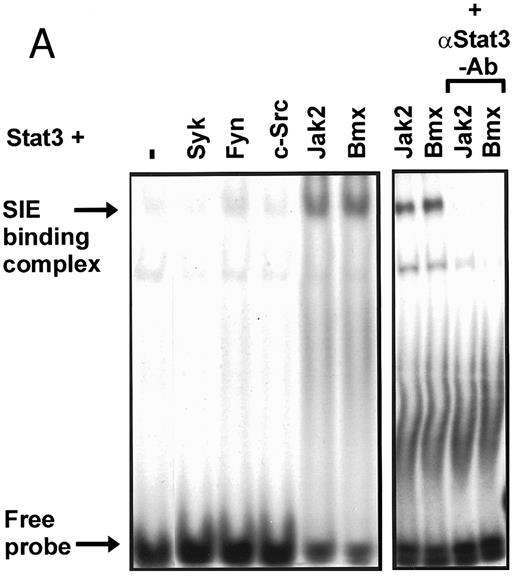
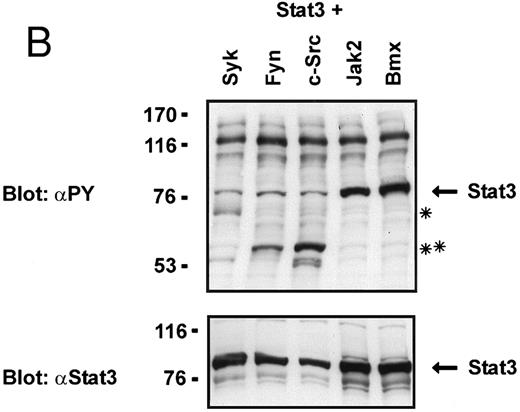
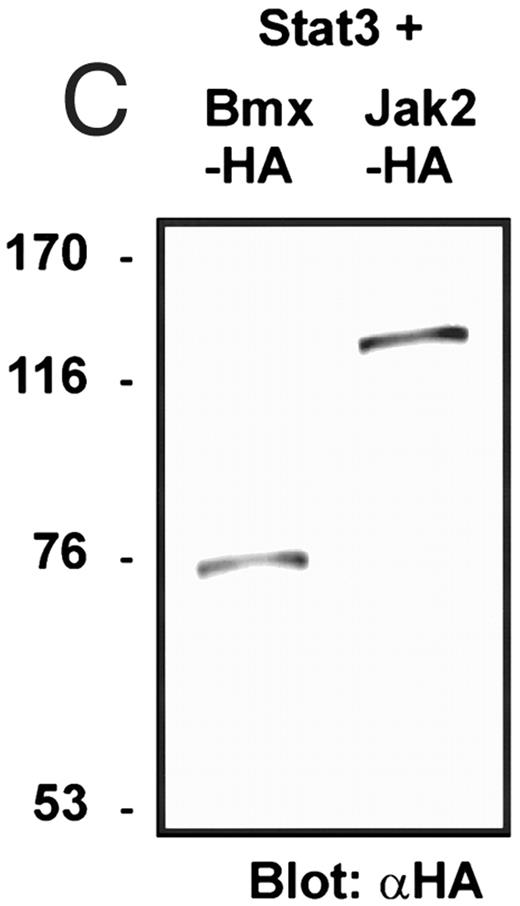
Coexpression of Bmx induces activation of Stat3. (A) COS cells were transfected with Stat3 expression vector together with Syk, Fyn, c-Src, Bmx-HA, or Jak2-HA expression vectors. Lysates were analyzed in the mobility shift assay using 33P-labeled SIE oligonucleotide. Lysates expressing Stat3 and Bmx-HA or Jak2-HA were supershifted using anti-Stat3 antibody. (B) The same lysates were electrophoresed in 7.5% SDS-PAGE and Western immunoblotted with anti-phosphotyrosine antibody or anti-Stat3 antibody. The autophosphorylated c-Src, Fyn, and Syk polypeptides are indicated by asterisks. (C) The Bmx-HA and Jak-HA containing lysates were also immunoblotted using anti-HA antibody.
Coexpression of Bmx induces activation of Stat3. (A) COS cells were transfected with Stat3 expression vector together with Syk, Fyn, c-Src, Bmx-HA, or Jak2-HA expression vectors. Lysates were analyzed in the mobility shift assay using 33P-labeled SIE oligonucleotide. Lysates expressing Stat3 and Bmx-HA or Jak2-HA were supershifted using anti-Stat3 antibody. (B) The same lysates were electrophoresed in 7.5% SDS-PAGE and Western immunoblotted with anti-phosphotyrosine antibody or anti-Stat3 antibody. The autophosphorylated c-Src, Fyn, and Syk polypeptides are indicated by asterisks. (C) The Bmx-HA and Jak-HA containing lysates were also immunoblotted using anti-HA antibody.
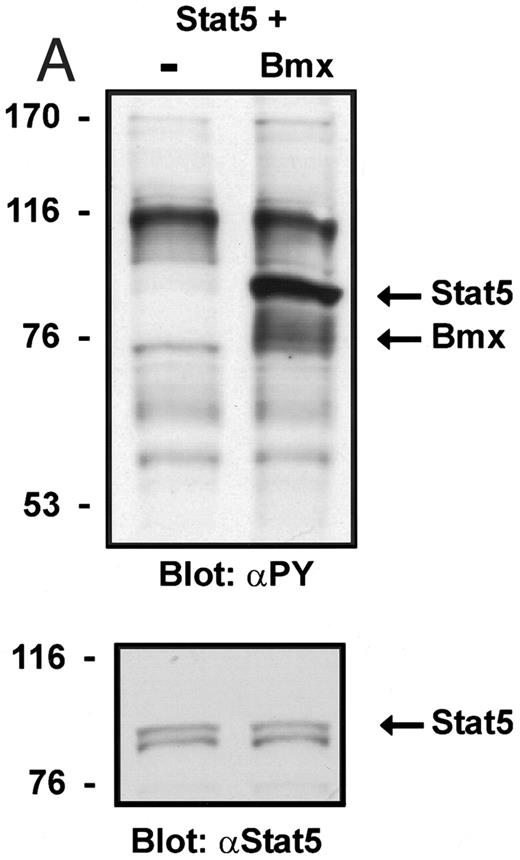
Coexpression of Bmx induces activation of Stat5. (A) COS cells were transfected with Stat5 expression vector alone or together with Bmx-HA expression vector. Cell lysates were electrophoresed in 7.5% SDS-PAGE and Western immunoblotted with anti-Stat5 and anti-phosphotyrosine antibodies. (B) The same lysates were analyzed in a mobility shift assay using 32P-labeled oligonucleotide from the promoter of the β-casein gene. The lysates were also supershifted with anti-Stat5 antibody.
Coexpression of Bmx induces activation of Stat5. (A) COS cells were transfected with Stat5 expression vector alone or together with Bmx-HA expression vector. Cell lysates were electrophoresed in 7.5% SDS-PAGE and Western immunoblotted with anti-Stat5 and anti-phosphotyrosine antibodies. (B) The same lysates were analyzed in a mobility shift assay using 32P-labeled oligonucleotide from the promoter of the β-casein gene. The lysates were also supershifted with anti-Stat5 antibody.
Stat transcription factors (signal transducers and activators of transcription) are essential mediators of cytokine-induced gene expression, and their activation is mediated through induction of tyrosine phosphorylation.26 Cytokines activate receptor-associated Jak tyrosine kinases (Jak1, Jak2, Jak3, and Tyk2), which are required for phosphorylation of receptor-recruited Stat transcription factors (Stats 1, 2, 3, 4, 5A, 5B, and 6).26 Tyrosine-phosphorylated Stats dimerize through their SH2 domains to form homodimers or heterodimers, which are subsequently translocated to the nucleus. In addition to tyrosine phosphorylation, serine phosphorylation of at least Stat1 and Stat3 is required for their optimal activity as transcriptional activators.27 Stats are critical mediators of the cytokine-specific responses, including differentiation of hematopoietic cells. For example, Stat4 is required for IL-12–mediated differentiation of Th1 cells, and Stat6 is crucial for the development of IL-4–dependent Th2 responses.28-31
The present study was undertaken to explore the cellular signaling mechanisms used by a Tec family tyrosine kinase, Bmx. Like the other Tec kinases, Bmx contains the PH, SH2, and kinase domains, whereas the short proline-rich region implicated in interactions with Src kinases is missing, and the SH3 domain does not conform to the consensus characteristics.4 Bmx was originally cloned from bone marrow cells and has recently been shown to be expressed early in myeloid differentiation and in myeloid leukemias.3 4 Therefore, Bmx could be involved in hematopoietic receptor signaling, and here we have investigated the possible role of the Bmx kinase in activation of the Stat signaling pathway.
MATERIALS AND METHODS
Cell culture and transfections. COS-7 and 293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; GIBCO BRL, Life Technologies, Gaithersburg, MD) and antibiotics. Transfections were done using the Calcium-Phosphate Transfection kit (GIBCO) according to manufacturer's instructions. The cells were obtained 72 hours after transfection for immunoprecipitation and electrophoretic gel mobility shift assay and after 48 hours for luciferase assay. Sf9-insect cells were grown in Sf900 medium (GIBCO) with 10% FBS (GIBCO) and antibiotics.
DNA constructs. Hemagglutinin (HA)-epitope was added to the 3′ terminus of Bmx cDNA4 in Bluescript SK (Stratagene, La Jolla, CA). A polymerase chain reaction (PCR) fragment of Bmx, containing the DNA sequence for the HA-epitope, was amplified using 5′-TAT GTC AGT TCA GTC GGA-3′ and 5′-CGT GTC GAC TCA AGC GTA ATC TGG AAC ATC GTA TGG GTA ATG CTT GTC TTT TTC CCG AAG-3′ oligonucleotides. This fragment was used to replace the 3′ BstXI-EcoRV fragment of Bmx in Bluescript SK. Full-length HA-tagged Bmx was cleaved out as a BssHII-Sal I fragment and cloned into Mlu I-Sal I sites of the pCI-Neo expression vector (Promega, Madison, WI), creating the Bmx-HA expression vector.
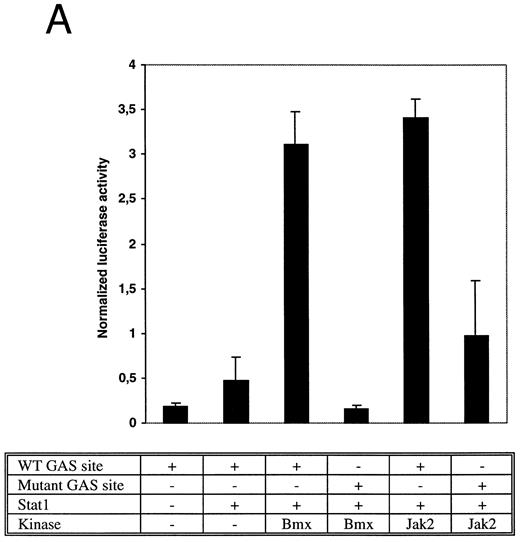
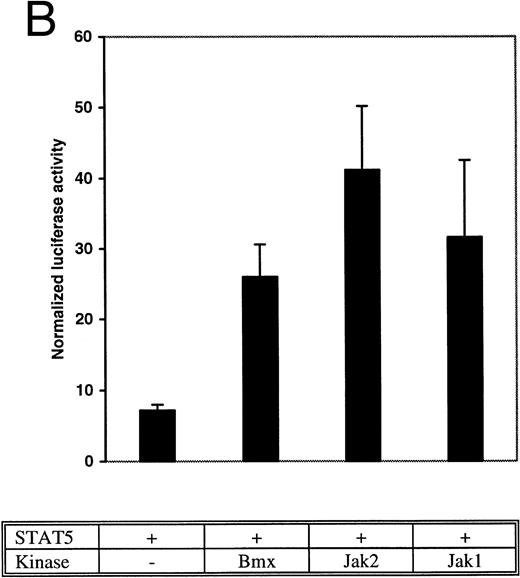
Bmx induces Stat-mediated transcription. (A) COS cells were transfected with Stat1α expression vector together with a luciferase reporter vector containing a wild-type (wt) or a mutant GAS-site (mut-GAS). In addition, cells were transfected with Jak2 or Bmx expression vectors. In control lysates cells were transfected with wild-type GAS luciferase construct alone or together with Stat1 expression vector. Cell lysates were analyzed for Stat1-dependent luciferase activity normalized to constitutively expressed luciferase activity from a cotransfected control vector (see Materials and Methods for details). The values shown are means from three independent experiments and standard errors of the mean. (B) COS cells were transfected with a luciferase reporter containing a Stat5 binding site from the promoter region of the Spi gene. Additionally the cells were transfected with Bmx-HA, Jak2-HA, or Jak1 expression vectors or with an empty vector as a control. Cell lysates were analyzed for Stat5-dependent luciferase activity as in (A).
Bmx induces Stat-mediated transcription. (A) COS cells were transfected with Stat1α expression vector together with a luciferase reporter vector containing a wild-type (wt) or a mutant GAS-site (mut-GAS). In addition, cells were transfected with Jak2 or Bmx expression vectors. In control lysates cells were transfected with wild-type GAS luciferase construct alone or together with Stat1 expression vector. Cell lysates were analyzed for Stat1-dependent luciferase activity normalized to constitutively expressed luciferase activity from a cotransfected control vector (see Materials and Methods for details). The values shown are means from three independent experiments and standard errors of the mean. (B) COS cells were transfected with a luciferase reporter containing a Stat5 binding site from the promoter region of the Spi gene. Additionally the cells were transfected with Bmx-HA, Jak2-HA, or Jak1 expression vectors or with an empty vector as a control. Cell lysates were analyzed for Stat5-dependent luciferase activity as in (A).
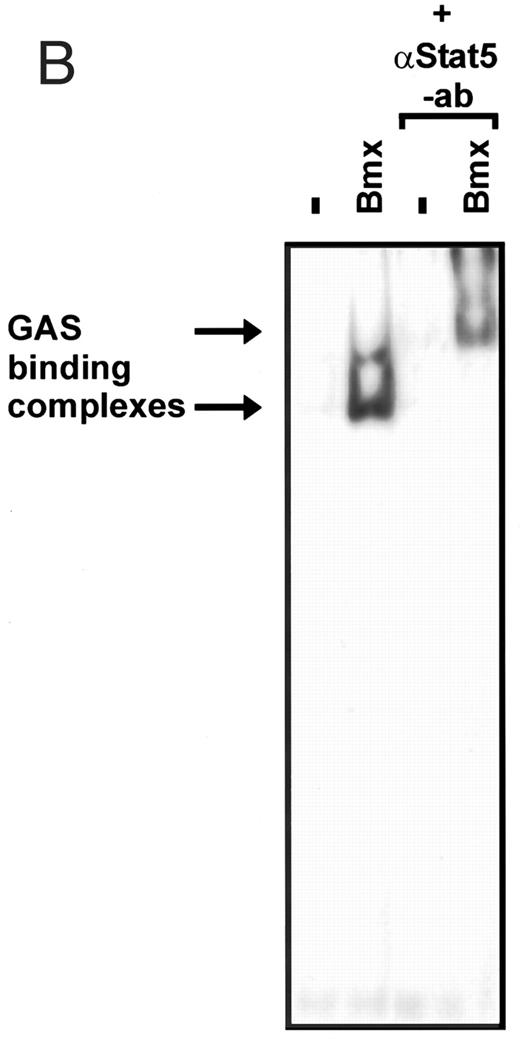
Induction of endogenous GAS-binding activity by Bmx expression. COS cells were transfected with Bmx, Jak2, or control expression vectors, as shown, and nuclear extracts of transfected cells were prepared. Endogenous Stat activation was analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. The same lysates were also incubated with anti-Stat1 antibody (ISGF3 [p91]) before mobility shift analysis with the IRF-1 GAS probe. As a control we analyzed a COS lysate containing overexpressed Stat1 and Bmx.
Induction of endogenous GAS-binding activity by Bmx expression. COS cells were transfected with Bmx, Jak2, or control expression vectors, as shown, and nuclear extracts of transfected cells were prepared. Endogenous Stat activation was analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. The same lysates were also incubated with anti-Stat1 antibody (ISGF3 [p91]) before mobility shift analysis with the IRF-1 GAS probe. As a control we analyzed a COS lysate containing overexpressed Stat1 and Bmx.
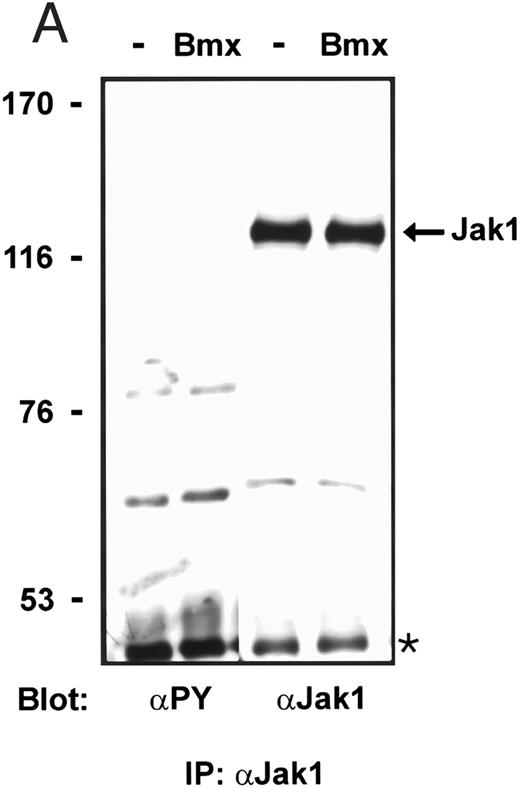
Bmx does not phosphorylate endogenous Jak kinases. (A) 293T cells were transfected with Bmx-HA expression vector or left without transfection. Jak1 was immunoprecipitated from cell lysates using anti-Jak1 antibody and the immunoprecipitates were electrophoresed in 6% SDS-PAGE followed by Western immunoblotting using anti-Jak1 or anti-phosphotyrosine antibodies. The Ig heavy chains are indicated by an asterisk. (B) Total cell lysates were electrophoresed in 6% SDS-PAGE followed by Western immunoblotting with anti-phosphotyrosine antibodies.
Bmx does not phosphorylate endogenous Jak kinases. (A) 293T cells were transfected with Bmx-HA expression vector or left without transfection. Jak1 was immunoprecipitated from cell lysates using anti-Jak1 antibody and the immunoprecipitates were electrophoresed in 6% SDS-PAGE followed by Western immunoblotting using anti-Jak1 or anti-phosphotyrosine antibodies. The Ig heavy chains are indicated by an asterisk. (B) Total cell lysates were electrophoresed in 6% SDS-PAGE followed by Western immunoblotting with anti-phosphotyrosine antibodies.
Induction of Stat3 tyrosine phosphorylation in insect cells. Sf9 cells were infected with Stat3 baculovirus alone or together with Bmx or Jak2 viruses or left uninfected. The cells were lysed 72 hours following infection and the lysates were immunoprecipitated using anti-Stat3 antibodies (A), anti-HA antibodies (Bmx) (B), or anti-Jak2 antibodies (C). The immunoprecipitates were electrophoresed in 7.5% SDS-PAGE, followed by Western immunoblotting using anti-phosphotyrosine antibodies, anti-Stat3 antibodies, anti-HA antibodies, or anti-Jak2 antibodies, as indicated. The anti-phosphotyrosine blot of Stat3-immunoprecipitate from Jak2 coinfected cells (A) is a longer exposure, as we repeatedly found weaker Stat3 tyrosine phosphorylation in Jak2 coinfections than in Bmx coinfections.
Induction of Stat3 tyrosine phosphorylation in insect cells. Sf9 cells were infected with Stat3 baculovirus alone or together with Bmx or Jak2 viruses or left uninfected. The cells were lysed 72 hours following infection and the lysates were immunoprecipitated using anti-Stat3 antibodies (A), anti-HA antibodies (Bmx) (B), or anti-Jak2 antibodies (C). The immunoprecipitates were electrophoresed in 7.5% SDS-PAGE, followed by Western immunoblotting using anti-phosphotyrosine antibodies, anti-Stat3 antibodies, anti-HA antibodies, or anti-Jak2 antibodies, as indicated. The anti-phosphotyrosine blot of Stat3-immunoprecipitate from Jak2 coinfected cells (A) is a longer exposure, as we repeatedly found weaker Stat3 tyrosine phosphorylation in Jak2 coinfections than in Bmx coinfections.
Induction of Stat1 tyrosine phosphorylation in insect cells. (A) Sf9 cells were infected with Stat1 baculovirus alone or together with Bmx or Jak2 viruses or left uninfected. The cells were lysed 72 hours following infection, and the lysates were immunoprecipitated using anti–ISGF-3(p91) antibodies. The immunoprecipitates were electrophoresed in 7.5% SDS-PAGE, followed by Western immunoblotting using anti-phosphotyrosine antibodies or anti-Stat1 antibodies as indicated. (B) Equal amounts of protein from the lysates used in (A) were electrophoresed in 7.5% SDS-PAGE and Western immunoblotted with anti-phosphotyrosine antibodies. The tyrosine phosphorylated Bmx and Jak2 kinases are indicated by asterisks.
Induction of Stat1 tyrosine phosphorylation in insect cells. (A) Sf9 cells were infected with Stat1 baculovirus alone or together with Bmx or Jak2 viruses or left uninfected. The cells were lysed 72 hours following infection, and the lysates were immunoprecipitated using anti–ISGF-3(p91) antibodies. The immunoprecipitates were electrophoresed in 7.5% SDS-PAGE, followed by Western immunoblotting using anti-phosphotyrosine antibodies or anti-Stat1 antibodies as indicated. (B) Equal amounts of protein from the lysates used in (A) were electrophoresed in 7.5% SDS-PAGE and Western immunoblotted with anti-phosphotyrosine antibodies. The tyrosine phosphorylated Bmx and Jak2 kinases are indicated by asterisks.
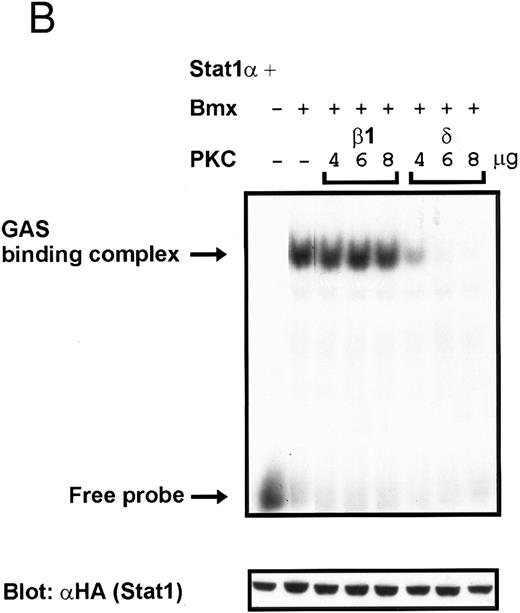
Coexpression of PKCδ inhibits Bmx-induced DNA binding of Stat1. (A) Bmx (3 μg) and Stat1α (4 μg) expression vectors were transfected into COS cells together with expression vectors for the indicated PKC isoforms (4 μg), as shown. Cell lysates were analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. First lane shows the 32P-labeled GAS oligonucleotide without cell lysate. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted for Stat1 using anti-HA antibodies. (B) The dose dependence of PKCδ inhibition of Stat1 DNA binding was evaluated by cotransfecting COS cells with expression vectors for Bmx (3 μg) and Stat1α (4 μg) and the indicated amounts of either PKCβ1 or PKCδ vectors. Cell lysates were analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted for Stat1 using anti-HA antibodies.
Coexpression of PKCδ inhibits Bmx-induced DNA binding of Stat1. (A) Bmx (3 μg) and Stat1α (4 μg) expression vectors were transfected into COS cells together with expression vectors for the indicated PKC isoforms (4 μg), as shown. Cell lysates were analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. First lane shows the 32P-labeled GAS oligonucleotide without cell lysate. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted for Stat1 using anti-HA antibodies. (B) The dose dependence of PKCδ inhibition of Stat1 DNA binding was evaluated by cotransfecting COS cells with expression vectors for Bmx (3 μg) and Stat1α (4 μg) and the indicated amounts of either PKCβ1 or PKCδ vectors. Cell lysates were analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE blotted for Stat1 using anti-HA antibodies.
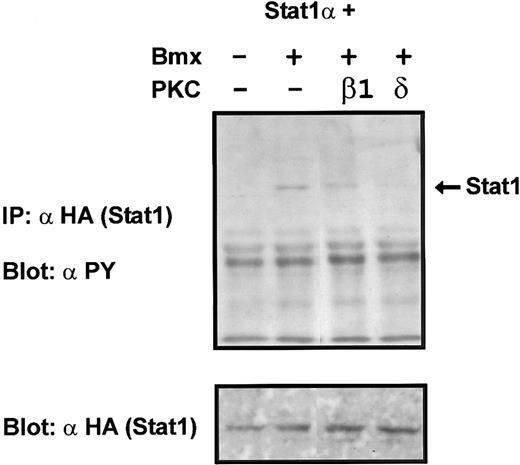
Coexpression of PKCδ inhibits Bmx-induced tyrosine phosphorylation of Stat1. Stat1α (4 μg) and Bmx (3 μg) expression vectors were transfected into COS cells together with the PKC vectors (4 μg). Stat1α was immunoprecipitated from cell lysates using anti-HA antibodies. The immunoprecipitates were separated in 7.5% SDS-PAGE, transferred to nitrocellulose membrane, and probed for anti-phosphotyrosine. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE probed for Stat1α using anti-HA antibodies.
Coexpression of PKCδ inhibits Bmx-induced tyrosine phosphorylation of Stat1. Stat1α (4 μg) and Bmx (3 μg) expression vectors were transfected into COS cells together with the PKC vectors (4 μg). Stat1α was immunoprecipitated from cell lysates using anti-HA antibodies. The immunoprecipitates were separated in 7.5% SDS-PAGE, transferred to nitrocellulose membrane, and probed for anti-phosphotyrosine. Shown in the lower panel is an immunoblot from total cell lysates electrophoresed in 7.5% SDS-PAGE probed for Stat1α using anti-HA antibodies.
Jak1 and Jak2 expression vectors have been previously described.32 33 HA-epitope was added to the 3′ end of Jak2 cDNA cloned into the RK5 expression vector. A PCR fragment containing the sequence for HA-epitope was obtained with oligonucleotides 5′-AAG GCC AGA AGG ATG CCC AGA T-3′ and 5′-TAG ATA TCG CGG CCG CCT AAG CGT AGT CAG GGA CGT CGT ACG GAT ACG CAG CTA TAC TGT CCC GGA-3′ and cloned into Bcl I-EcoRV sites of Jak2 in RK5. The HA-epitope–tagged Jak2 was cloned as a Sal I-EcoRV fragment into Sal I-Sma I sites of pCI-Neo vector, creating the Jak2-HA expression vector.
Expression vectors for different PKC isoforms34-37 and for c-Src and Fyn kinases32 have been previously described. Luciferase reporter constructs with wild-type and mutated gamma interferon–activated sequence (GAS) oligonucleotides,38 the Spi luciferase vector,39 and expression vectors for Syk,40 HA-epitope–tagged Stat1α,41 Stat3,42 and Stat5A43 were kind gifts from Drs Richard Pine, Gunnar Norstedt, Tomas Mustelin, Chris Schindler, James N. Ihle, and Berndt Groner, respectively, and have all been previously described.
Jak2-expressing baculovirus was constructed by cloning Jak2 cDNA into pVL1392 and transfecting it together with baculovirus DNA into Sf9 insect cells. Bmx-expressing baculovirus was constructed by cloning the HA-tagged Bmx cDNA into pBluebac4 (Invitrogen, San Diego, CA) and using the Bac-N-Blue Transfection Kit (Invitrogen) for creating the virus. The Stat1 and Stat3 baculoviruses were kind gifts from Dr James N. Ihle.
Antibodies. Anti–phosphotyrosine antibody (clone 4G10) was from Upstate Biotechnology (Lake Placid, NY). Polyclonal anti-Jak2 antibody was described previously.32 Anti–HA-epitope antibody (clones 12CA5 and 16B12) was from Berkeley Antibody Company (Richmond, CA). Monoclonal anti-Stat1(p91), anti–ISGF-3(p91), anti-Stat3, and anti-Jak1 antibodies were from Transduction Laboratories (Lexington, KY). Polyclonal anti-Stat5 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoprecipitation and Western blotting. For immunoprecipitation, cells were lysed in Triton lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 10% glycerol, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X100, 50 mmol/L NaF, 1 mmol/L Na3VO4 ) supplemented with phenylmethylsulfonyl fluoride (PMSF ), aprotinin, leupeptin, and pepstatin A or in boiling sodium dodecyl sulfate (SDS) lysis buffer (0.5% SDS, 0.05 mmol/L Tris-HCl, pH 8, 1 mmol/L dithiothreitol (DTT), 50 mmol/L NaF, 1 mmol/L Na3VO4 ) for immunoprecipitation of Stat3. The SDS lysates were diluted with 1.5× RIPA buffer (1.25% NP-40, 1.25% sodium deoxycholate, 12.5 mmol/L NaH2PO4 , pH 7.2, 2 mmol/L EDTA, 50 mmol/L NaF, 1 mmol/L Na3VO4 with protease inhibitors) before immunoprecipitation. Immunoprecipitation was performed by incubating with specific antibodies for 2 to 3 hours followed by incubating with protein-A-Sepharose (Sigma, St Louis, MO) or with protein-G-Sepharose (Pharmacia, Uppsala, Sweden) for 1 to 1.5 hours. Immunoprecipitates were washed four times with Triton lysis buffer followed by elution with reducing Laemmli sample buffer. Immunoprecipitates, and samples of total cell lysates, were separated in SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membrane (Micron Separation Inc, Westborough, MA). Equal amounts of protein from cell lysates were always used for immunoprecipitations and Western blotting of total cell lysates. Protein concentrations of the lysates were measured using the BioRad Protein Assay system (Bio-Rad Laboratories, Hercules, CA). Immunodetection was performed using specific primary antibodies, biotinylated anti-mouse or anti-rabbit secondary antibodies (Dako A/S, Denmark), and streptavidin-biotin horseradish peroxidase–conjugate (Amersham Life Science, UK) followed by ECL (Amersham) or using specific primary antibodies and HRP-conjugated anti-mouse or anti-rabbit secondary antibodies (Amersham) and ECL detection (Amersham).
Electrophoretic gel-mobility shift assay. For gel-mobility shift assay, cells were lysed in WCE lysis buffer (50 mmol/L Tris-HCl, pH 8, 0.5% NP-40, 10% glycerol, 0.1 mmol/L EDTA, 150 mmol/L NaCl, 50 mmol/L NaF, 0.5 mmol/L Na3VO4 ) supplemented with protease inhibitors. Eight micrograms of protein from total cell lysate was used in each mobility shift reaction. Annealed GAS oligonucleotide (GAS site of murine IRF-1 gene, 5′-CTA GAG CCT GAT TTC CCC GAA ATG ATG AG-3′) or high-affinity SIS inducible element (SIE) oligonucleotide (5′-GAT CAG CAT TTC CCG TAA ATC CC-3′) was end-labeled by T4 polynucleotide kinase using [γ-32P]adenosine triphosphate (ATP) or [γ-33P]ATP (Amersham). A total of 0.05 ng of 32P-labeled oligonucleotides or 0.5 ng of 33P-labeled oligonucleotides was used per reaction. Specifically, cell lysates and 200 ng poly-dI-dC (Pharmacia) were incubated on ice for 15 minutes followed by additional 15-minute incubation after adding labeled GAS/SIE oligonucleotide. Reactions were analyzed in 4.5% Tris Borate EDTA (TBE)-PAGE (2.2× TBE concentration) followed by autoradiography. For supershift analysis, WCE lysates were incubated with specific antibodies 30 minutes on ice before adding poly-dI-dC and labeled oligonucleotides. Stat5 gel-mobility shift assay was performed as described above except that annealed oligonucleotide corresponding to the Stat5 binding site in the β-casein gene promoter (5′-AGATTTCTAGGAATTCAAATCC-3′) was used in the binding reactions. Twenty-four femtomoles of probe labeled by end-filling with Klenow and [α32P]dCTP (Amersham) was used in each reaction, and the reactions were analyzed in 4.5% TBE-PAGE (0.25× TBE concentration).
Nuclear extracts were prepared as described earlier.44 In brief, cells were lysed in buffer A (50 mmol/L HEPES, pH 7.5, 100 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 2 mmol/L Na3VO4 , 2 mmol/L sodium molybdate, 2 mmol/L EDTA [PSB buffer], 0.2% NP-40, 10 mmol/L magnesium chloride, and protease inhibitors) and centrifuged. The nuclei were washed once with buffer B (same as A without NP-40), and finally lysed in buffer C (PSB with 0.1% NP-40, 0.3 mmol/L NaCl, 10% glycerol, and protease inhibitors). Nuclear extracts (6 μg), poly-dI-dC (240 ng/μL), bovine serum albumin (BSA; 1.5 mg/mL) and 32P-labeled GAS oligonucleotide (0.05 ng) were incubated 30 minutes at room temperature, and the reactions were resolved in 4.5% TBE (0.25×) PAGE, followed by autoradiography. For supershift analysis, nuclear extracts were incubated with anti–ISGF3 antibodies (0.25 μg) 40 minutes on ice before adding poly-dI-dC, BSA, and labeled GAS oligonucleotide.
Luciferase assay. For luciferase assay, cells were transfected with Stat-dependent reporter constructs together with a pRLTK control vector constitutively expressing Renilla luciferase (Promega). Cells were lysed in Passive lysis buffer (Promega), and luciferase activity of the lysates was determined using Dual-Luciferase Reporter Assay System (Promega) according to manufacturer's instructions. The Stat-dependent luciferase activity was normalized to constitutively expressed Renilla luciferase activity.
RESULTS
Activation of Stat signaling pathway by the Bmx kinase. The downstream signaling events for the Bmx tyrosine kinase were investigated by studying its possible role in activation of the Stat signaling pathway, which is used by multiple hematopoietic receptors. The activating receptor system for the Bmx kinase is currently not known, but the expression of Bmx in myeloid cells suggests its participation in hematopoietic receptor signaling.3
Overexpression of Bmx in COS cells resulted in its tyrosine phosphorylation, which was weakly visible as an approximately 76-kD tyrosine phosphorylated band in an anti-phosphotyrosine immunoblot of total cell lysates (Fig 1C). Immunoprecipitation of HA-epitope–tagged Bmx with anti–HA antibody followed by Western immunoblotting with anti-phosphotyrosine or in vitro immunocomplex kinase assay clearly showed that the immunoprecipitated Bmx was tyrosine phosphorylated and catalytically active (not shown).
To explore the ability of Bmx to activate Stat proteins, Bmx and Stat1 were coexpressed in COS cells. As positive controls we expressed Stat1 with Jak1 or Jak2, which have previously been shown to tyrosine phosphorylate different Stat proteins.1 Lysates from transfected cells were analyzed by immunoprecipitation of HA-epitope–tagged Stat1 using anti–HA-antibody, followed by anti-phosphotyrosine immunoblotting. Figure 1A shows that Stat1 became tyrosine phosphorylated in Bmx-transfected cells, as well as in Jak1- and Jak2-transfected cells. The control blot shows equal Stat1 expression in Bmx/Jak transfected lysates.
Induction of Stat1 DNA binding activity requires phosphorylation of a single C-terminal tyrosine residue.1 To investigate if Bmx-induced tyrosine phosphorylation was occurring at this activating tyrosine residue of Stat1, we analyzed the DNA binding activity of Bmx, Jak1, or Jak2 phosphorylated Stat1 in an electrophoretic mobility shift experiment (Fig 1B). The lysates were incubated with a 32P-labeled oligonucleotide representing the GAS site of the murine IRF-1 gene, which binds activated Stat dimers, including Stat1 homodimers. The Bmx kinase was found to induce retardation of the GAS oligonucleotide as efficiently as the Jak1 and Jak2 kinases (Fig 1B). When coexpressed with Stat3, Bmx was found to induce retardation of the labeled high-affinity SIE DNA sequence, a high-affinity binding site for Stat3 (Fig 2A). Bmx also induced tyrosine phosphorylation of Stat3 as shown in Fig 2B, and when coexpressed with Stat5, Bmx induced the tyrosine phosphorylation and DNA binding activity of Stat5 (Fig 3A and B). Figure 2C shows equal expression of HA-epitope–tagged Bmx and Jak2 kinases in the cell lysates analyzed in Fig 2A and B.
To evaluate the specificity of Bmx-induced Stat activation, we studied the ability of other cytoplasmic tyrosine kinases to induce Stat DNA binding activities in coexpression experiments with different Stat proteins in COS cells. Syk and the Src family kinases, Fyn and c-Src, showed much weaker activity in the mobility shift analysis of Stat1 (Fig 1C) and Stat3 (Fig 2A). In addition, overexpression of Syk, c-Src, and Fyn resulted in only weak induction of Stat3 tyrosine phosphorylation, whereas tyrosine phosphorylation of Stat3 was prominent in Bmx- and Jak2-expressing cells (Fig 2B). Anti-phosphotyrosine immunoblots of equal amounts of protein from total cell lysates revealed tyrosine phosphorylated bands corresponding to autophosphorylated Syk, Fyn, and c-Src kinases, confirming the expression and activation of these kinases in the cells (Figs 1C and 2B). The appearance of additional tyrosine phosphorylated bands in longer exposures also indicated tyrosine kinase activity of the transfected kinases toward endogenous substrates (not shown). This indicates that the ability to activate Stats is not merely correlated with overall kinase activity, and therefore, Stat proteins cannot be regarded as common substrates for cytoplasmic tyrosine kinases.
The DNA binding activity of Stat1 is not sufficient for optimal induction of Stat1-mediated transcriptional activation. The carboxy truncated splice variant Stat1β, which lacks the C-terminal serine phosphorylation site, binds DNA following interferon gamma (IFN-γ) stimulation, but does not activate transcription.27 Therefore, we wanted to explore if activation of Bmx was sufficient to stimulate Stat1-dependent transcription. Stat1 and Bmx or Stat1 and Jak2 expression vectors were transfected into COS cells together with luciferase constructs containing either wild-type or mutant IRF-1 GAS site upstream of a thymidine kinase minimal promoter. An internal standard was used to normalize luciferase activity to the transfection efficiency. Bmx was found to activate transcription through the wild-type GAS site as efficiently as Jak2, but not through the mutant GAS site (Fig 4A). We also studied whether Bmx was able to induce Stat5-dependent transcription using a reporter construct of the promoter region of the Serine protease inhibitor (Spi) gene, which binds activated Stat5 dimers.39 COS cells were transfected with this reporter construct together with Stat5 and Bmx, Jak1 or Jak2 expression vectors. The results in Fig 4B show that Bmx induced Stat5 transactivation similarly to Jak1 or Jak2.
Activation of endogenous Jak/Stat proteins by Bmx expression. We also explored whether Bmx was able to activate endogenous Stat proteins. Expression of Bmx alone in COS cells was found to induce GAS binding activity of endogenous Stat proteins to a similar extent as expression of Jak2 (Fig 5). By supershift experiments the GAS binding complex induced by both Jak2 and Bmx was found to be composed of Stat1 proteins. We also tested whether similar expression of Bmx induced activation of endogenous Jak kinases. 293 T cells transfected with Bmx were lysed and immunoprecipitated using anti-Jak1 antibody, followed by Western immunoblotting using anti-Jak1 or anti-phosphotyrosine antibodies (Fig 6). We could not detect Jak1 tyrosine phosphorylation either when Jak1 was immunoprecipitated from nontransfected control cells or cells transfected with Bmx expression vector. This suggests that expression of Bmx does not activate endogenous Jak1, and that Bmx-induced Stat activation is mediated independently of Jak kinases.
Activation of Stat proteins by Bmx in insect cells. To explore the ability of Bmx to induce activation of Stat proteins also in insect cells, we infected Sf9 cells with Stat3 baculovirus either alone or together with Bmx or Jak2 viruses. The baculovirus-expressed Bmx and Jak2 kinases were found to be tyrosine phosphorylated in insect cells (Fig 7B and C). Tyrosine phosphorylation of Stat3 in coinfected cells was analyzed by immunoprecipitation of Stat3, followed by anti-phosphotyrosine immunoblotting. The results indicated that Bmx was able to induce Stat3 activation efficiently also in insect cells (Fig 7A). Similarly, we analyzed tyrosine phosphorylation of Stat1 induced by Bmx or Jak2 coinfection in insect cells (Fig 8). Figure 8A shows that both kinases induced tyrosine phosphorylation of Stat1.
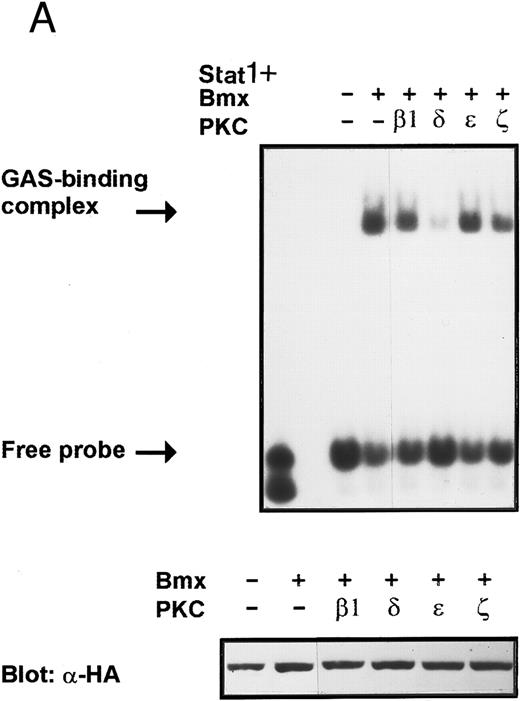
Inhibition of Bmx-induced Stat1 activation by PKCδ. Activation of PKC by phorbol esters has been found to inhibit certain cytokine-mediated responses.45,46 In addition, several PKC isoforms have been found to interact with the Btk and Itk kinases modifying their kinase activity.18 25 These reports indicate that PKC may regulate cytokine-induced signaling and particularly may modulate signaling mediated by Tec family kinases. Therefore, we wanted to investigate possible regulation of the Bmx kinase by different PKC isoforms. COS cells were transfected with expression vectors for Stat1 and Bmx together with expression vectors for either β1, δ, ε, and ζ PKC isoforms. The lysates were analyzed in a mobility shift assay using labeled IRF-1 GAS sequence as a probe. The Bmx-induced DNA binding activity of Stat1 was found to be inhibited by PKCδ, whereas the β1, ε, and ζ PKC isoforms had no effect (Fig 9A). Expression of the different PKC isoforms was confirmed by Western immunoblotting of total cell lysates using specific anti-PKC antibodies (not shown). To examine the specificity of the inhibitory effect of PKCδ, we analyzed the dose-dependent effect of PKCδ and PKCβ1 on Bmx-induced Stat1 DNA binding activity (Fig 9B). PKCδ inhibited the Bmx-induced Stat1 DNA binding already at the lowest concentration tested, whereas PKCβ1 had no effect even when it was transfected over two times more than Bmx.
PKCδ inhibits tyrosine phosphorylation of Stat1. Because tyrosine phosphorylation is required for the induction of DNA binding activity of Stat proteins, inhibition of Stat tyrosine phosphorylation was a potential mechanism for the inhibitory effect of PKCδ. However, tyrosine phosphorylation is not always sufficient for induction of Stat DNA binding activity. For example, in certain human cancer cell lines, IFN-α–induced gene expression has been found to be inhibited by proteins preventing the DNA binding of activated ISGF-3 complexes.47 48 To analyze these possibilities, COS cells were transfected with Stat1 and Bmx expression vectors together with PKCβ1 or PKCδ vectors. The cell lysates were analyzed by immunoprecipitation of Stat1 and Western immunoblotting with anti-phosphotyrosine. PKCδ, but not PKCβ1, was found to inhibit Bmx-induced tyrosine phosphorylation of Stat1 (Fig 10), indicating that the lack of Stat1 DNA binding activity in PKCδ coexpressions was caused by inhibition of Stat1 tyrosine phosphorylation.
DISCUSSION
In this study we have investigated the signaling mechanisms used by Bmx, a member of the Tec tyrosine kinase family. We analyzed the role of Bmx in activation of Stat transcription factors by studying the Bmx-induced Stat tyrosine phosphorylation and DNA binding to GAS or SIE DNA elements, which specifically bind different Stat factors. Expression of Bmx in COS cells induced endogenous GAS-binding activity, and when coexpressed, Bmx induced the DNA binding activity and tyrosine phosphorylation of Stat1, Stat3, and Stat5. Furthermore, we found that overexpression of Bmx was sufficient to induce transcriptional activation of Stat1- and Stat5-dependent reporter genes to the same extent as overexpression of Jak1 or Jak2. The specificity of Bmx-induced Stat activation was analyzed by comparing it with Stat activation induced by other cytoplasmic tyrosine kinases. Overexpressed Syk, c-Src, or Fyn showed much weaker ability to activate Stats as compared with Bmx or Jak2, illustrating clear differences in their substrate specificity. Also, Tec has been found neither to phosphorylate purified Stat1 in an in vitro immunocomplex kinase assay nor to induce tyrosine phosphorylation or DNA binding activity of Stat1 in insect cells.49 The various Tec family members appear to display clear differences in their substrate recognition, because we found that Bmx activated Stat1, Stat3, and Stat5 as efficiently as Jaks both in insect and mammalian cells. Based on these results, Stat activation is a likely downstream signaling event for the Bmx kinase also in vivo. The receptor system where Bmx is activated is not known yet, but it is interesting to note that Bmx was cloned from a bone marrow cDNA library, and we have detected Bmx RNA in CD34+ cells from cord blood as well as in granulocytes and myeloid leukemias.3
One possible explanation for Bmx-induced Stat activation is that Bmx induces activation of endogenous Jak kinases. However, two findings suggest that Bmx directly phosphorylates Stat proteins to activate them. First, we have not detected endogenous Jak activation in Bmx transfected cells (Fig 6), although Bmx is able to induce activation of DNA binding of endogenous Stat proteins (Fig 5). Furthermore, the transfected Bmx and Jak kinases were equally effective in induction of Stat activation. This would be unlikely if Bmx works through activation of an endogenous Jak kinase.
Previous studies indicate that besides the Jak kinases, other protein kinases can also directly activate Stat proteins. For example, receptors for epidermal growth factor (EGF ) and platelet-derived growth factor have been found to directly phosphorylate Stat proteins.49-52 In addition, engagement of the T-cell antigen receptor in purified peripheral blood T lymphocytes leads to activation of Stat1 and Stat3 without any detectable Jak activation.53 It is interesting to note that Stat activation has recently been found to occur within minutes after activation of the B-cell antigen receptor, although the Jak kinases have not been found to be associated with the antigen receptor.54 Therefore, it is reasonable to assume that in addition to Jaks, other tyrosine kinases may regulate the Stat pathway.
Enhanced activation of Stat proteins has been observed in cells transformed by v-Src, suggesting that v-Src directly activates Stat factors.55,56 Previous studies showed that overexpression of v-Src in NIH 3T3 cells activated Stat3, whereas overexpression of c-Src resulted in much weaker Stat3 activation.55 Stat1 was not activated in these experiments, which is in accordance with our results (Fig 1C). In longer exposures of our mobility shift and Western blot gels, we could also detect Stat1 and Stat3 activation by c-Src, but this activation was much weaker than that seen by Bmx or Jak kinases. One explanation is that activation of Src kinases is weaker in COS cells compared with strong activation of v-Src observed in fibroblastic cell lines. In our experiments the activity of c-Src as indicated by the magnitude of cellular tyrosine phosphorylation induced by c-Src expression was comparable with that induced by Bmx or Jak kinases. It should be noted that under physiological situations, such as stimulations with growth factors or cytokines, the level of Src activation and cellular phosphorylation is rather low.
Chaturvedi et al have recently shown that overexpression of v-Src in a myeloblastic cell line induces constitutive activation of Stat1, Stat3, and Stat5, and they suggest that only Stat3 is activated directly by v-Src.57 Furthermore, in the myeloid system v-Src is unable to induce constitutive Jak activation, although v-Src induces activation of Jak1 and Jak2 in NIH 3T3 fibroblasts.58 These results indicate that overexpression of the v-Src kinase has different effects on the Jak/Stat-signaling pathway in different cell types. Also, endogenous cytokine receptors use different Jaks and Stats in a cell type–specific manner.
Constitutive Stat activation has been also detected in myeloid and lymphoid leukemia cells.59,60 In Ph1 positive leukemia cell line, K562 constitutive activation of Stat5 has been shown to occur independently of Jak kinases, most probably through Bcr/Abl.61,62 Bmx is expressed in acute and chronic myeloid leukemia cells, and it will be of interest to study the possible role of Bmx in Stat activation in these cells.3
Activation of PKC commonly occurs in signal transduction through hematopoietic transmembrane receptors, and several isoforms of PKC have been found to regulate Tec family kinases.18,25 We found that PKCδ, but not β1, ε, or ζ isoforms, inhibited the Bmx-mediated Stat1 activation by preventing tyrosine phosphorylation of Stat1. PKCδ has recently been found to be involved in many protein tyrosine phosphorylation–mediated signaling events. In these situations PKCδ often becomes tyrosine phosphorylated, which modulates its kinase activity. For example, substance P–, carbacol-, EGF-, and IgE-induced signaling pathways have been found to induce tyrosine phosphorylation of PKCδ, and activation of the Fcε-receptor by IgE alters the substrate specificity of PKCδ.63-65 These reports thus provide evidence for interactions between tyrosine kinase signaling and enzymes of the PKC family, particularly PKCδ. We also found that coexpression of Bmx with PKCδ induced tyrosine phosphorylation of PKCδ, indicating possible regulation of PKCδ by Bmx (unpublished data of authors and Dr Imre Västrik, 1996).
The mechanism by which PKCδ inhibits Bmx-induced Stat activation is currently unknown, but several possibilities exist. First, PKCδ may downregulate the activity of Bmx by directly interacting with Bmx or through another PKCδ-activated kinase. Alternatively, PKCδ may activate a phosphatase capable of dephosphorylating Stats or Bmx. We are further analyzing these possibilities. However, we have not detected any differences in Bmx tyrosine phosphorylation or kinase activity on cotransfection of Bmx and PKCδ (unpublished data of the authors and Dr Imre Västrik, 1996). PKC-mediated, protein tyrosine phosphatase activation has previously been found to regulate Stat signaling pathways. For example, IFN-α signaling in primary monocytes has been found to be inhibited by phorbol esters through an as yet uncharacterized protein tyrosine phosphatase.46 Phorbol esters have also been found to increase the catalytic activity and expression of the SH2-containing phosphatase, SHP-1, which is implicated in the downregulation of cytokine receptor signaling by dephosphorylating the Jak kinases.66-70
In conclusion, activation of the Bmx tyrosine kinase was found to lead to activation of Stat transcription factors. The Jak kinases are essential for Stat activation through cytokine receptors, but in noncytokine receptors Stat activation does not require Jak activation. In these instances other tyrosine kinases must be responsible for Stat activation.
ACKNOWLEDGMENT
We thank Dr Eija Korpelainen for helpful discussions, Drs Imre Västrik and Elena Arighi for help and collaboration, and Drs Berndt Groner, James N. Ihle, Tomas Mustelin, Gunnar Norstedt, Richard Pine, and Chris Schindler for kindly providing the reagents specified in Materials and Methods.
Supported by the Academy of Finland, Finnish Cancer Society, and Sigrid Juselius Foundation.
Address reprint requests to Olli Silvennoinen, Department of Virology, Haartman Institute, Haartmaninkatu 3, PO Box 21, FIN-00014 University of Helsinki, Helsinki, Finland.

![Fig. 5. Induction of endogenous GAS-binding activity by Bmx expression. COS cells were transfected with Bmx, Jak2, or control expression vectors, as shown, and nuclear extracts of transfected cells were prepared. Endogenous Stat activation was analyzed in the mobility shift assay using 32P-labeled IRF-1 GAS oligonucleotide. The same lysates were also incubated with anti-Stat1 antibody (ISGF3 [p91]) before mobility shift analysis with the IRF-1 GAS probe. As a control we analyzed a COS lysate containing overexpressed Stat1 and Bmx.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/11/10.1182_blood.v90.11.4341/3/m_bl_0014f5.jpeg?Expires=1766534267&Signature=4r4rE-XPC70cAHAiLuHuCpZK4mOF99J-goXZOobESixSJ0IcJ4DQ4RyanQgDM93aLgLcj20kbkAKoxxUsDMl~u4U7WtEWpcluvEPkxhmnCxDsv1a2dCsFlgy1DWX5gV-i-xJ8lQP9arIGC24QCIu6pO~U0qIT1ptVANlaJsgIs~Sc2a5XJbSXD-uG7-6NwfYq0ujAYX7Io07gRCCEEfS-uMsrJ~hnyb0IKwuoqtQLtVFUatnPNlY--C9J6083jOQEWjZsWXTRtrK4NgPn6FwsdVpbvdzsziXXN-38prdu6mBn~YV2Uaxtd58VbCDzKRLJA~ZhkR2WlQ99C7KMWQUOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)