“It seems more than ever likely that blood-derived stem cells will replace marrow for many indications ... ”
J. Goldman, Bone Marrow Transplant 14:1, 1994
RECENT DATA from the International Bone Marrow Transplant Registry (IBMTR) showed that since 1990 the number of autologous transplants has exceeded that of allogeneic transplant. Furthermore, blood cells have been used more often than bone marrow (BM) in autologous transplants since 1993 (IBMTR newsletter July 1995). This trend mirrors that reported by the Europe Blood and Marrow Transplant Group (EBMT)1 and the Australian Bone Marrow Transplant Registry (ACCORD September 1995, The Newsletter from the Australian Coordinating Committee on Organ Registries and Donation). According to EBMT data, the number of autologous transplants in lymphoma and breast cancer in Europe has increased fivefold between 1990 and 1994. In this period the percentage of autologous transplants using blood cells increased from 15% to 75%.
The regular use of blood cells for transplant started only in the mid 1980s,2-6 although there were a few earlier sporadic case reports.7-10 This lagged behind the use of BM, the more intuitive source, for 15 years, and yet it is now the predominant cell source for hemopoietic rescue. For the early history of autologous blood cell transplant, readers are referred to an excellent chapter by two pioneers in this field, Korbling and Fliedner.11
Blood progenitor cell mobilization in humans was initially noted during recovery after myelosuppressive chemotherapy.12-17 The ability of recombinant hematopoietic growth factors to mobilize blood cells, either alone18-20 or by enhancing chemotherapy mobilization,21-26 increased the use of mobilized blood cell for transplantation. Malignant contamination of mobilized blood cells received scant attention initially,9,27 but it has recently received more notice.28-32 The abundance of CD34+ cells in mobilized blood cells also lends itselves to gene therapy33,34 and ex vivo processing.35-38 However, little is known about the mechanism of mobilization of normal and malignant cells39,40 and the design of mobilization protocols remains empirical. Different approaches to study the quantitation of hematopoietic reconstitutive capacity in mobilized blood cells have been proposed,41-46 but little is known about the immune reconstitutive capacity.47-49 In the allogeneic setting, mobilized blood cells have also been used instead of BM.50 Umbilical cord or placental blood cells are another source of hematopoietic cells for transplantation51 but are outside the scope of this review. The emerging biology of mobilized blood cells and its increasing clinical use for hematopoietic rescue prompt this review.
The questions to be addressed in this review include the following: what are the molecular and humoral events leading to the mobilization of hematopoietic progenitors cells, how should the nature and quantity of the mobilized cells be measured, which are the effective blood cell mobilizers, when is the optimal time to obtain blood cells, how many blood progenitor cells are required for rapid reconstitution, what are the clinical and economical benefits of blood cell transplants, are allogeneic blood cells a feasible alternative to BM, and is there a place for mobilized blood cells in the treatment of nonmalignant diseases?
HISTORICAL PERSPECTIVE
Blood cell transplant is one of the few recently introduced treatment modalities where a reverse of the usual progression from animal studies to clinical trials occurs. Apart from a few initial animal studies,52-57 the major systematic developments in blood progenitor cell mobilization and transplantation occurred in the clinical arena.
Early experimental hematopoietic transplantation studies were mostly performed with murine BM rather than blood cells because of the inherent limitations to adequate and repeated sampling of blood from a small mammal-like mouse. In addition, steady-state blood cells were shown to be inferior to BM as a source of marrow repopulating cells.54 In humans progenitor cells are present in low quantities in blood during steady-state hematopoiesis postnatally,58-61 except for myeloproliferative states.62 The failure of engraftment in early reports of steady-state blood cell transplants in humans7,8 and clinical results of autologous transplantation using steady-state blood cells where hematopoietic reconstitution was no better than BM63,64 reinforced the emphasis on BM as the source of cells for hematopoietic rescue. These data overshadowed the more encouraging reports in larger animal models such as dogs53,57,65,66 and primates.55 Early hematopoietic recovery after chemotherapy was reported in a patient who received peripheral blood (PB) collected by leukaphereses from an identical twin.67 Another report of successful use of syngeneic blood cells for immune reconstitution appeared in 1972.68 Despite these studies the use of mobilized blood cells for transplantation was initially viewed with a healthy scepticism because mobilized blood cells were considered no different from steady-state blood cells.
Several developments contributed to a shift from steady-state to mobilized blood cells.
The increase of circulating progenitor cells following the administration of dextran sulfate to dogs and of endotoxin and adrenocorticotrophin to normal subjects, and after strenuous physical exercise was described as early as the 1970s.69 70 There was only a twofold to fourfold increase and the levels returned to normal within hours, so such transient mobilizations did not offer much scope for blood progenitor cell harvesting. Nonetheless, these observations indicated that progenitor cells could be mobilized to blood.
An improved colony-forming unit granulocyte-macrophage (CFU-GM) assay provided more accurate information on progenitor cell mobilization. A plating concentration of 1 to 2 × 105 PB mononuclear cells/mL was more optimal for the growth of PB progenitors than the 5 to 10 × 105/mL previously used because it takes into account the effect of monocytes in vitro.71
The feasibility of progenitor cell harvesting by leukapheresis was first demonstrated in normal subjects in 1980.72 Then successive reports of clinical studies showing the phenomenon of blood progenitor cell mobilization and the hematopoietic reconstitution advantage of mobilized blood cells2,4,6,15,17,20-26,73-76 followed. Systematic experimental animal studies with mobilized blood cells have since confirmed findings of clinical studies.77-79 Currently almost all blood cell transplants other than cord blood transplants are performed with mobilized blood cells.
THE PHENOMENON OF HEMATOPOIETIC PROGENITOR CELL MOBILIZATION
Hematopoietic chimerism after liver transplantation suggests that extramedullary transplantable progenitor cells exist,80 but whether they contribute to the mobilizable pool is not known. The general belief is that mobilized blood progenitor cells originate from BM. This postulate predicts a sequence of critical events during progenitor cell mobilization: firstly, modulation of the progenitor cell:BM stroma interaction, then the directed migration toward marrow sinuses and eventually egress through the basement membrane and the endothelial layer.
The localization of hematopoiesis to the BM involves developmentally regulated adhesive interactions between primitive hematopoietic cells and the stromal-cell–mediated hematopoietic microenvironment.81-88 This and the broad range of agents that can result in transient increases in blood progenitor cells89 led many investigators to propose that mobilization involves a perturbation of the adhesive interactions with stromal elements which, under steady-state conditions, are responsible for the physiologic retention of primitive hematopoietic progenitor cells in the BM.40,90-92 Primitive hematopoietic progenitor cells exhibit a wide range of cell adhesion molecules (CAMs) including members of the integrin, selectin, immunoglobulin superfamily and CD44 families of adhesion molecules. Ligands for many of these CAMs are expressed by BM stromal cells.82,85 86
The role and relative contribution of the many individual CAM-ligand pairs in the homing, lodgement, and retention of primitive hematopoietic progenitor cells within the BM remain largely unknown. However, recent studies have suggested an important contribution is made by the β-1-integrin VLA-4 whose two ligands, fibronectin and VCAM-1, are constitutively expressed by the marrow stroma.87,88,93-96 Notably, perturbation of VLA-4 function after administration of function-blocking anti–VLA-4 antibody to nonhuman primates was found to induce mobilization of hematopoietic progenitor cells.90 In the same study the investigators showed that antibody to β-2-integrin (CD18), which is also expressed by hematopoietic progenitor cells, failed to induce mobilization (at least over the time course measured), further emphasising the importance of the role of VLA-4 in restricting hematopoietic progenitor cells to the BM under steady-state conditions.
Evidence is now emerging that CAM may be involved in the process of mobilization. Several studies have shown that, relative to their counterparts in steady-state BM, CD34+ cells mobilized by a variety of regimens and cytokines consistently demonstrate reduced expression of certain CAMs, in particular VLA-4, LFA-1, and LFA-3, whereas others such as CD31, CD44, and CD62L remain unchanged.40,91,97 It is also important to note that functional changes in CAMs may occur without changes in surface expression. In common with mature leukocytes, CD34+ cells in steady-state BM express the β-1 integrins VLA-4 and VLA-5 in an inactive or low-affinity state.98,99 After treatment with a range of cytokines including interleukin-3 (IL-3), GM colony-stimulating factor (GM-CSF ), and stem cell factor (SCF ), CD34+ cells exhibit transient (within 10 to 15 minutes) dose-dependent increases in VLA-4 and VLA-5 ligand binding properties followed by a return to basal activation states.99-101 Whether such functional changes occur with other CAMs such as CD44,102 which do not exhibit significant changes in expression on mobilized progenitor cells, remains to be determined. Nonetheless, these data suggest that mobilization of hematopoietic progenitor cells may, at least in part, result from cytokine-induced changes in integrin function on CD34+ cells that facilitate their egress from the BM. However, it should be noted that the rapidity and transience of the change in adhesive properties observed in vitro after treatment of hematopoietic progenitor cells with cytokines are not in accord with the kinetic of hematopoietic progenitor cell release in vivo following administration of cytokines, which in the case of G-CSF, for example, occurs after several days.20
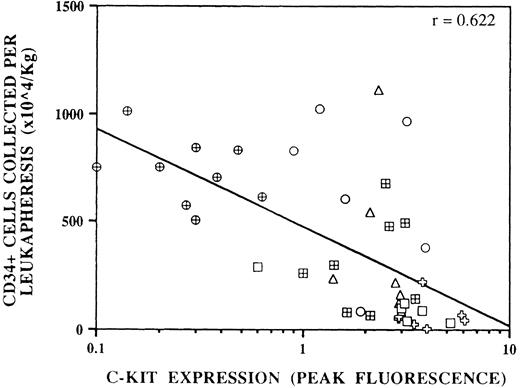
One of the most intriguing observations to emerge from the flow cytometric analysis of CD34+ cells in mobilized PB is the markedly reduced expression of c-kit on these cells compared with the levels on steady-state BM and PB CD34+ cells.39,40,91 Importantly, this occurred in all six mobilization protocols studied40 and the reduced expression is inversely correlated with progenitor cell yield (Fig 1). The mechanism responsible for this reduction in c-kit expression remains unknown, although the change in c-kit antigen density occurs in the BM before the egress of hematopoietic progenitor cells into the circulation.40 However, the interaction between c-kit and its ligand SCF, which exists both as a soluble form and as a membrane-bound form found on stroma cells, may be a paradigm of how cytokines, CAM, and marrow stroma influence the progenitor cell function. Cynshi et al103 have previously shown that in mice with Steel or WW mutations (that lack the ability to produce soluble SCF or have a defective c-kit receptor, respectively), G-CSF is far less efficient at mobilizing blood progenitor cells than in wild-type mice. Thus, mobilization of blood progenitor cells in response to G-CSF may in fact be a G-CSF + (endogenous) SCF response.
The correlation between c-kit downregulation on mobilized CD34+ cells and CD34+ cell yield (×106/kg BW) of a 10-L apheresis performed on the same day in the authors' institution. Forty-two specimens from six mobilization protocols were analyzed. (□), Patients who received cyclophosphamide 4 to 7 g/m2 intravenously and had blood cells harvested during recovery from myelosuppression. (✙), Patients who received IL-3 and GM-CSF at 5 μg/kg/d subcutaneously and had blood cells harvested during GM-CSF administration. (⊞), Chemotherapy naive patients who received G-CSF at 12 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7. (○, ▵), Patients who received myelosuppressive chemotherapy and G-CSF or GM-CSF and had blood cells harvested during recovery from myelosuppression. (⊕), Chemotherapy naive patient who received G-CSF at 12 μg/kg subcutaneously daily and SCF at 5 to 15 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7 of G-CSF administration. The correlation between c-kit downregulation and CD34+ cell yield per apheresis is significant at P << .005.
The correlation between c-kit downregulation on mobilized CD34+ cells and CD34+ cell yield (×106/kg BW) of a 10-L apheresis performed on the same day in the authors' institution. Forty-two specimens from six mobilization protocols were analyzed. (□), Patients who received cyclophosphamide 4 to 7 g/m2 intravenously and had blood cells harvested during recovery from myelosuppression. (✙), Patients who received IL-3 and GM-CSF at 5 μg/kg/d subcutaneously and had blood cells harvested during GM-CSF administration. (⊞), Chemotherapy naive patients who received G-CSF at 12 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7. (○, ▵), Patients who received myelosuppressive chemotherapy and G-CSF or GM-CSF and had blood cells harvested during recovery from myelosuppression. (⊕), Chemotherapy naive patient who received G-CSF at 12 μg/kg subcutaneously daily and SCF at 5 to 15 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7 of G-CSF administration. The correlation between c-kit downregulation and CD34+ cell yield per apheresis is significant at P << .005.
Primitive progenitor cells were capable of shape change and motility in vitro.104 However, factors modulating directed migration have not been defined. Furthermore, proteolytic activity possibly involving metalloproteases is presumably required for egress through the basement membrane. Although neutrophil motility and tissue migration have been well studied,105 no data are available on whether the same biochemical pathways and cytoskeleton are present or used in mobilized progenitor cells.
Although most studies investigating mechanisms of mobilization of hematopoietic progenitor cells have focused on changes in the properties of these cells, little work has been done to investigate the potential contributions of the marrow stroma to this phenomenon. Damage to the stroma from previous exposure to chemotherapy may, in part, explain the quantitatively poor mobilization frequently seen in some patients17,106 and also in murine models.78 Similarly, the increased stromal cell proliferation seen in patients receiving IL-3 may result in enhanced retention of hematopoietic progenitor cells106 107 and may therefore explain the relatively poor efficacy of such protocols.
Understanding the mechanism of mobilization may contribute to the development of more predictable and efficacious mobilization protocols. In particular, elucidation of the immediate molecular and biochemical events leading to dehesion and migration may allow us to mobilize progenitor cells more directly. Changes in integrin and selectin function have also been described in association with tumor cell trafficking108; hence, it may be possible to develop protocols that favor normal hematopoietic cell mobilization and reduce the risk of malignant contamination.
MOBILIZATION PROTOCOLS
Myelosuppressive Chemotherapy
Myelosuppressive chemotherapy was the first clinically useful blood progenitor cell mobilization protocol.3,4,15 During the recovery phase after myelosuppressive chemotherapy, 50-fold or more increases in PB CFU-GM occur. The extent of this increase seems to be proportional to the severity and duration of the cytopenia.3,16,25 High-dose cyclophosphamide is the most commonly reported protocol4,17,109 110 because it is active against most tumors and can be given justifiably even in diseases when conventional treatment is not sufficiently myelosuppressive.
The main limitations of chemotherapy mobilization are neutropenic sepsis, bleeding diathesis, and the unpredictability of the timing of apheresis. In a series of 60 patients receiving high-dose cyclophosphamide, hospitalization because of sepsis occurred in 44% to 100%, platelet transfusion in 18%, and death in 3% of the patients.109 Similar toxicity was reported by Kotasek et al110 and Jagannath et al.111 With the advent of hematopoietic growth factors it is no longer necessary to use myelosuppressive chemotherapy alone for mobilization.
Myelosuppressive Chemotherapy + Hematopoietic Growth Factors
Numerous phase II studies have suggested most strongly that the addition of hematopoietic growth factors such as G-CSF and GM-CSF to myelosuppressive chemotherapy enhances mobilization while reducing myelotoxicity.22,24,25 112-114
G-CSF.Schwartzberg26 described the effect of G-CSF in enhancing chemotherapy mobilization in a study involving 382 patients with various malignancies. They received either 4 g/m2 cyclophosphamide (HDC), HDC and 600 mg/m2 etoposide (HDCE), HDCE with G-CSF (6 μg/kg/d), or HDCE and 105 mg/m2 cisplatin (HDCEP) with G-CSF. Both dose escalation and addition of G-CSF were associated with higher CD34+ cell yield. The addition of G-CSF led to a doubling of mononuclear cell yield, but a fourfold to sixfold increase in CD34+ cell yield from 1.4 × 106/kg in HDCE to 6.6 × 106/kg in HDCE + G-CSF and 8.6 × 106/kg in HDCEP + G-CSF. Over half of patients in the last group achieved 20 × 104 CFU-GM/kg after one leukapheresis whereas only 40% of the HDC group reached this target after six leukaphereses. In an earlier study Schwartzberg et al25 reported a mean of 3 days shortening of neutropenia and a reduction of hospitalization from 33% in the chemotherapy-alone group to 8% to 20% in the chemotherapy + G-CSF group.
The dose of G-CSF used with chemotherapy is in the range of 3 to 6 μg/kg/d,24,25 usually started on the day after chemotherapy and continued until the completion of leukapheresis. It has become an increasingly common practice to use a 300-μg ampoule of G-CSF per day instead of adhering to a strict per-kilogram formula.24,115 The daily G-CSF dose is lower than the 10 to 24 μg/kg/d when used alone,20,116 but the duration required is longer at 8 to 12 days compared with 4 to 6 days, so there is no cost saving. However, a recent report described 42 patients who received 3 to 4 g/m2-cyclophosphamide and 300 μg G-CSF from day +5.115 Thirty-eight patients proceeded to transplant receiving a median 4.3 × 106 CD34+ cells/kg and achieved the expected rapid neutrophil and platelet reconstitution. Hence, a delayed start of G-CSF may reduce cost without loss of efficacy, as described with GM-CSF.
GM-CSF and IL-3.GM-CSF was the first cytokine shown to enhance blood progenitor cell mobilization by chemotherapy.112 Although its efficacy seems comparable with G-CSF, it is now less commonly used than G-CSF, probably because of its side effects such as fever, hypoxemia, and first-dose effect. The dose is usually 5 μg/kg/d or 250 μg/m2/d subcutaneously. Because higher doses are associated with more side effects they are rarely used. Starting GM-CSF 5 days after chemotherapy is just as effective as starting on day 1, but starting on 7 or 10 days after chemotherapy may be less effective.
A protocol of sequential IL-3 and GM-CSF after chemotherapy gave a higher yield of progenitor cells compared with GM-CSF following chemotherapy or chemotherapy alone.113 Both IL-3 (days 1 to 5) and GM-CSF (days 6 to 14) were used at 250 μg/m2/d.
PIXY321.Preliminary data suggest that PIXY321 (GM-CSF/IL-3 fusion protein) at 500 to 1,000 μg/m2 after chemotherapy also enhances mobilization. An ongoing randomized study comparing PIXY321 (750 μg/m2) with G-CSF (5 μg/kg/d), GM-CSF (5 μg/kg/d), or G- + GM-CSF (2.5 μg/kg/d) administered after cyclophosphamide 3 g/m2 suggests that more CFU-GM and megakaryocytic progenitors are mobilized by PIXY321 than by other cytokines,117 although the differences are no more than onefold to twofold. Another study suggests that PIXY321 enhancement of cyclophosphamide mobilization is similar to GM-CSF.118
Many different myelosuppressive chemotherapy protocols have been used in conjunction with cytokines for mobilization. None stand out, so it is more important that mobilization be incorporated as part of therapy. Another emerging impression is that adequate mobilization may occur even with chemotherapy regimens that are only mildly myelotoxic, such as cyclophosphamide at 1-2 g/m2.114
Hematopoietic Growth Factors Alone
G-CSF.G-CSF stimulates neutrophil granulopoiesis in a dose-dependent manner and increases the level of PB progenitor cells in cancer patients.18 Dose escalation beyond 10 to 16 μg/kg/d does not appear to further enhance mobilization. The minimum dose is yet to be defined. The level of PB progenitor cells increases 40- to 80-fold after 4 to 5 days of treatment.20,119 On cessation of G-CSF progenitor cell levels return to baseline values within 4 to 6 days.120 Syngeneic transplants in mice showed that G-CSF mobilized blood cells have substantial numbers of primitive stem cells capable of long-term hematopoietic reconstitution and repopulating the thymus.77 In humans, PB CD34+ cells mobilized with G-CSF contain subsets of CD38−, HLA-DR−, and CD33− cells and are capable of generating CFU-GM in liquid culture for 3 weeks or more.39
Sheridan et al20 were the first to report the hematopoietic reconstitutive capacity of G-CSF–mobilized blood cells. Seventeen patients with poor-prognosis nonmyeloid malignancy received G-CSF at 12 μg/kg/d subcutaneously for 6 days and had leukapheresis performed on days 5, 6, and 7. The CFU-GM levels shared a median increase of 58-fold. CFU-GM yield was highest on day 5, with a mean total yield of 33 ± 6 × 104/kg body weight (BW), range 0.8 to 90 × 104/kg BW. Fourteen patients were given both the cryopreserved blood cells and autologous BM cryopreserved before mobilization. Compared to two historical groups transplanted with autologous BM with and without posttransplant G-CSF, the most significant finding was a more rapid platelet reconstitution with a reduction in platelet transfusion requirement.
Bensinger et al121 described 12 patients administered G-CSF at 16 μg/kg/d subcutaneously. The number of PB CD34+ cells increased 10-fold over baseline values, peaking at about day 5 of G-CSF therapy. Neutrophil and platelet reconstitution were both more rapid than with historical control patients who received autologous BM with or without posttransplant cytokines.
Eighty-five patients with relapsed Hodgkin's disease received autologous hematopoietic rescue with steady-state or G-CSF–mobilized blood cells, with or without BM.122 They were assigned to ‘no growth factor during mobilization or following infusion’ (group 1, n = 32), ‘GM-CSF following infusion’ (group 2, n = 21), ‘G-CSF following infusion’ (group 3, n = 7), or ‘G-CSF for mobilization and following infusion’ (group 4, n = 20). The CFU-GM yield and the rate of platelet and neutrophil reconstitution were highest in group 4. This study shows clearly the advantage of G-CSF–mobilized blood cells over steady-state blood cells and BM.
Basser et al123 described G-CSF mobilization in patients who had not received previous chemotherapy. Three leukaphereses yielded 115 × 104 CFU-GM/kg BW, range 23 to 274 × 104/kg BW, much higher than that in patients who had previous chemotherapy.20 Notably the progenitor cell yield, though higher, still showed a 10-fold variation.123
In allogeneic blood cell transplantation G-CSF was used for mobilization in almost all reported cases and the apheresis target is generally set at ≥3 × 106 CD34+ cells/kg BW. The most common dose of G-CSF used is 10 μg/kg BW/d subcutaneously and most centers perform leukapheresis after 4 to 5 days of G-CSF.124-128 A dose-dependent CD34+ cell mobilization by G-CSF between 3 and 10 μg/kg BW/d has been observed,129 but further data suggest that higher doses of G-CSF, such as 10 to 12 μg/kg twice daily, give a higher yield than once daily.130 One leukapheresis is often sufficient, but there were donors from whom low numbers of CD34+ cells were collected.
The side effects of G-CSF are few. Bone pain and asymptomatic elevation of serum alkaline phosphatase and GGT are the most common side effects. The latter does not require treatment while analgesia is usually adequate for the former. Two forms of G-CSF are currently available. Filgrastim is the nonglycosylated form from Escherichia coli, while lenograstim is the glycosylated form from Chinese hamster ovary (CHO) cells. Lenograstim was shown to be more active than filgrastim on a weight-by-weight basis in BM assay in vitro, although they were equivalent by immunologic assays.131 In postchemotherapy neutropenia, lenograstim at 150 μg/m2 was claimed to be as effective as filgrastim at 5 μg/kg.132 Very few comparisons in mobilization are available, although a recent abstract suggested that lenograstim at 10 μg/kg/d gives a better mobilization of CD34+ cells and CFU-GM than filgrastim.133 This is based on a cross-over study in 32 healthy male volunteers with a minimum 4 weeks' wash-out period. The PB progenitor cell levels were 30% higher during lenograstim administration. Whether the formulation of G-CSF influences its mobilization capacity remains an open question. Nonetheless, the hematopoietic reconstitutive capacity of G-CSF–mobilized blood cells is beyond doubt.
G-CSF/SCF.SCF, ligand to c-kit, has only a modest effect on hematopoietic progenitor cell proliferation in vitro but exerts potent synergistic effects with direct-acting hematopoietic growth factors. In baboons a 10- to 100-fold increase in CD34+ cells, CFU-GM, and burst-forming units-erythroid (BFU-E) as well as detectable levels of multilineage progenitor cells (CFU-Mix) and high proliferative potential progenitor cells (HPP-CFC) occurred in blood after 200 μg/kg/d of SCF administered either subcutaneously or intravenously.134 These SCF-mobilized blood cells rescued lethally irradiated baboons.135 Its mobilization effect on humans when used alone has not been published, although it has been reported in meetings that patients rescued with SCF-mobilized blood cells showed delayed engraftment and required back-up BM.
The clinical use of SCF + G-CSF for mobilization followed the demonstration of synergy of such a combination in experimental animals. Administration of low-dose SCF + G-CSF to mice and baboons mobilized greater numbers of progenitor cells compared with G-CSF alone.136,137 Genetic marking showed that a higher proportion of mice transplanted with SCF + G-CSF mobilized blood cells remain stably reconstituted by donor cells, compared with mice transplanted with blood cells mobilized by G-CSF alone.79
In patients with poor-prognosis stage II/III breast cancer with no previous chemotherapy, concomitant SCF enhanced mobilization by G-CSF.138 In eight patients who received SCF 10 μg/kg/d concomitantly with G-CSF, both the mean PB CFU-GM level on day 6 and the leukapheresis yield were 70% higher than the cohort receiving G-CSF alone.
The same group suggested that mobilization with SCF/G-CSF is schedule dependent. In patients receiving G-CSF alone, the level of PB CFU-GM returned to normal within days of G-CSF cessation. In patients receiving SCF + G-CSF, PB CFU-GM remained elevated 5 days later at 8,816/mL. In eight patients receiving 3 days of SCF before 7 days of combined SCF + G-CSF the levels 5 days after cessation of study drugs were even higher, at 22,338/mL.139 Hence, further studies on the scheduling of the SCF/G-CSF may have a great potential for harvesting extremely high numbers of progenitor cells.
The synergism between SCF and G-CSF was also seen in patients who had previous chemotherapy.140 One hundred eleven women with stage II-IV breast cancer received either G-CSF 10 μg/kg/d alone or with SCF at 10-25 μg/kg/d administered concomitantly. The CD34+ cell yield showed a significant SCF dose response. Patients receiving 20 or 25 μg SCF/kg/d showed a 2.5- and 4-fold higher yield (8.1 and 13.6 × 106/kg BW) than those receiving G-CSF alone or with 10 μg SCF/kg/d (3.2 and 2.6 × 106/kg BW).
SCF administration has produced an anaphylactic type reaction in occasional subjects, so current protocols are usually administered under H-1 and H-2 blockade and α and β adrenergic cover. This may result from mast cell activation and may limit its use in atopic subjects.
GM-CSF.In the same year that Duhrsen et al reported G-CSF's blood progenitor cell mobilization effect, Socinski et al19 described an 18-fold increase in PB CFU-GM after 3 to 7 days of GM-CSF at 4 to 64 μg/kg/d as a continuous intravenous infusion. Later reports described a more modest fourfold to ninefold increase in PB CFU-GM.141-143 Haas et al141 transplanted 6 patients with GM-CSF–mobilized blood cells. Of the 5 evaluable patients, the median time to reach 0.5 × 109 neutrophils/L and 20 × 109 platelets/L were 28.5 and 39 days, respectively. This engraftment rate was similar to that following BM rescue, not surprising in view of the low CFU-GM dose of <1 × 104/kg BW.141 Peters et al144 also suggested that GM-CSF is less efficacious than G-CSF in mobilization. Although GM-CSF has been approved for mobilization in the United States, G-CSF is used more frequently.
IL-3.IL-3 has a proliferative effect on primitive hematopoietic cells in vitro145 and gives rise to a moderate increase in leukocyte and platelet counts on in vivo administration,146 but has little activity in mobilization.147 In rhesus monkeys IL-3 (33 μg/kg/d subcutaneously for 11 to 14 days) followed by GM-CSF (5.5 μg/kg/d for 5 to 14 days) was compared with either GM-CSF or IL-3 alone.148 There was a 63-fold increase in PB CFU-GM over steady-state levels in the sequential protocol compared with 12- and 14-fold increases, respectively, in the other protocols. Unfortunately, side effects limit the dose of IL-3 in humans to 5 to 10 μg/kg/d. In humans a phase I/II study of blood progenitor cell mobilization with IL-3/GM-CSF (at 5 μg/kg/d each) showed only a modest yield of CFU-GM 28 ± 8 × 104/kg BW and 1.8 ± 0.6 × 106 CD34+ cells/kg BW.106 IL-3 has also been combined with G-CSF but it is not clear how much it improves mobilization with the latter. There is no evidence that cytokines active on primitive cells such as IL-3 and SCF can mobilize more progenitor cells in patients with a limited hematopoietic reserve or that they can mobilize a different spectrum of hematopoietic progenitors.
PIXY321.Ghielmini et al149 reported a phase I/II study of PIXY321 administered daily at doses of 500 to 1,000 μg/m2/d subcutaneously over 14 days to nine patients before chemotherapy. A modest biphasic increase in neutrophil counts occurred, accompanied by an increased BM cellularity. There was a modest 3- to 10-fold increase in the number of myeloid and erythroid colony-forming cells in blood. The mobilized cells were not able to sustain hematopoiesis in long-term liquid culture to the same degree as BM.
Flt3 ligand alone or with G-CSF/GM-CSF.Flt3 ligand has been shown to be a costimulatory factor for the proliferation of primitive lymphohematopoietic progenitors in vitro and is able to protect mice from lethal irradiation. Brasel et al150 reported preliminary data from a murine study comparing the progenitor cell mobilization effect of Flt3 ligand at 10 μg/d, G-CSF at 2 μg/d, and GM-CSF at 1 μg/d, administered intraperitoneally for 9 days. Flt3 ligand produced an 83-fold increase of PB CFU-GM after 9 days but Flt3 ligand + G-CSF gave a 2,193-fold increase. Synergism with GM-CSF was present but minimal. BFU-E and CFU-Mix were also mobilized. The length of administration required and the synergistic effect with G-CSF both suggest that other intermediary cytokines are involved.
Macrophage inflammatory protein-1α (MIP-1α).MIP-1α inhibits primitive stem cell proliferation and has been investigated as a myeloprotective agent during chemotherapy. Murine studies with BB10010, a genetically engineered variant of human MIP-1α showed that a single subcutaneous injection caused a twofold increase in circulating CFU-S and cells with marrow repopulating ability (MRA). Although G-CSF increased the circulating levels of CFU-S and MRA 20- to 30-fold, a single injection of BB10010 after 2 days of G-CSF increased the levels 30- and 100-fold, respectively.151 No published data on human subjects are available.
Other Agents
Human erythropoietin and IL-6 have both been shown to have very modest effect in progenitor cell mobilization.152,153 IL-1 at 1 μg produced a 30-fold increase in CFU-GM and a 10-fold increase in CFU-S12 in PB after 4 to 8 hours, and these murine PB cells have long-term hematopoietic reconstitutive capacity.154 Unfortunately IL-1 is unlikely to be used in humans because of its toxicity even at very low doses. The same group also demonstrated that 30 μg of IL-8 injected intraperitoneally in Balb/C mice produced a 17-fold increase in CFU-GM after 15 minutes, but the level of CFU-GM returned to normal after 60 minutes. Transplant studies showed that IL-8–mobilized cells had lymphohematopoietic repopulating ability.155 The immediate time course of mobilization is of major interest because it suggests that IL-8 may modulate the immediate molecular and biochemical events leading to mobilization. A 20-fold increase in PB CFU-GM was described 5 days after cessation of 5 days of IL-2 at 3 × 106 μg/m2/d, but whether that is a result of BM recovery is not known.156 The mobilization potential of thrombopoietin has not been reported.
FACTORS AFFECTING YIELD
In progenitor cell mobilization after myelosuppressive chemotherapy, the dose of chemotherapy, the severity of myelosuppression, and the rate of recovery of leukocyte count all correlate positively with progenitor cell yield.16,17,26,109,110 The addition of G-CSF or GM-CSF to myelosuppressive chemotherapy appears to enhance progenitor cell yield,26,157 158 although no phase III studies have been reported.
In all categories of mobilization protocols described above the amount of previous chemoradiotherapy and the degree of BM involvement are significant determinants of progenitor cell yield. This may be measured as the number of cycles of chemotherapy,24,158,159 the duration of previous chemotherapy,17,160 previous wide-field radiotherapy,113,161 the interval between previous chemotherapy and mobilisation,162 exposure to stem cell toxic drugs such as BCNU and melphalan,161 and the higher yields in allogeneic donors.
These variables are probably all indicators of hematopoietic reserve. Therefore, it is noteworthy that Fruehauf et al163 reported that PB CD34+ cells and colony-forming cells during steady-state hematopoiesis are measures of a patient's mobilizable pool after G-CSF and chemotherapy. Their analysis of 15 patients showed that a level of ≥0.4 × 106 PB CD34+ cells/L at steady-state predicts with 95% probability that 2.5 × 106 CD34+ cells/kg would be collected with six leukaphereses. The correlation between BM CD34+ is much less significant and the investigators suggested that there are mature CD34low cells in BM that do not circulate. However, several major technical problems limit the use of BM progenitor cell levels as an indicator of hematopoietic reserve.
The level of BM progenitor cells can only be expressed as a percentage of total cells in the aspirate, but the degree of blood dilution in the aspirate varies significantly according to the technique used and the volume aspirated.164 Hence, the levels of BM progenitor cells in a BM aspirate which includes PB as well as BM cells may not be a real estimate of the true incidence of progenitor cells in BM. The cellularity of BM is also different in different parts of the body; eg, sternal aspirates are usually more cellular than iliac spine aspirates,165,166 and the volume of total body BM remains an estimate.167 Furthermore, these problems are aggravated in disease states.164,167 These variables make it very difficult to correlate the incidence of progenitor cells in a BM aspirate with the number of progenitor cells in the body.165 In contrast, if there is an equilibrium between the progenitor pools in PB and BM then the level of steady-state PB progenitor cells may be a more accurate indicator of hematopoietic reserve than the incidence of progenitor cells in a BM aspirate. Nothdurft et al65 found that the number of CFU-GM infused correlated significantly with hematopoietic reconstitutive capacity in beagle dogs transplanted with steady-state syngeneic or autologous PB. Hence, steady-state PB progenitor levels may be a reliable, albeit indirect, indicator of pluripotent stem cells. However, the stringency required to measure low levels of progenitor cells in steady-state hematopoiesis by flow cytometry or clonogenic assays mean that only laboratories with well-standardized assays could use this index.
Sequential progenitor cell mobilization in the same patient provides a unique setting for analyzing the efficacy of mobilization uncomplicated by patient, disease, and prior treatment variables. In a study of patients undergoing two mobilizations within 3 months the progenitor cell yields were the same when the same dose of chemotherapy was used twice, higher when 7 g/m2 cyclophosphamide was used compared with 4 g/m2, and also higher when chemotherapy + GM-CSF was used instead of a IL-3 + GM-CSF combination.168
Experimental data suggest that chemoradiotherapy damages stem cells169,170 and progressive delay in recovery after successive chemotherapy cycles is an experience common to most hemato-oncologists. The importance of hematopoietic injury may be indicated by the rate of posttransplant recovery, which is significantly affected by the amount of chemotherapy received before transplant conditioning.171 The importance of hematopoietic reserve in progenitor cell mobilization underscores how critical it is to incorporate mobilization as part of planned treatment rather than as part of salvage for resistant disease.172,173 This is particularly relevant given that hematopoietic growth factors are used increasingly as an adjunct to chemotherapy treatment because this may result in earlier and more severe stem cell exhaustion.173
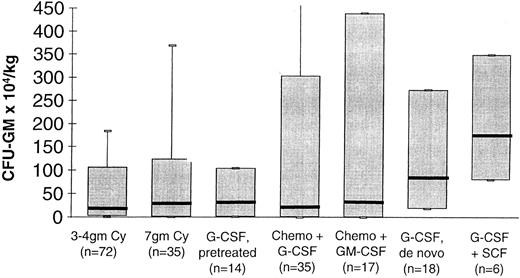
Figure 2 shows the progenitor cell yields from different mobilization protocols observed in the authors' institution over the last 10 years. Stringent in-house quality control measures assured the reliability of CFU-GM and CD34+ cell measurements throughout the period. Patient recruitment into these groups was not randomized so the groups were not strictly comparable. However, the data indicate strongly that the dose of mobilizing chemotherapy, the synergism between chemotherapy and G-CSF/GM-CSF, the synergism between G-CSF and SCF, and a healthy BM are all predictors of a high progenitor cell yield.
The CFU-GM yield of different mobilization protocols used in the authors' institution. ‘3-4gm Cy’ denotes patients who received cyclophosphamide 3 to 4 g/m2 intravenously and had blood cells harvested during recovery from myelosuppression. ‘7gm Cy’ denotes patients who received cyclophosphamide 7 g/m2 intravenously and had blood cells harvested during recovery from myelosuppression. ‘G-CSF, pretreated’ denotes patients who received G-CSF at 12 μg/kg subcutaneously and had blood cells harvested on days 5, 6, and 7. ‘Chemo + G-CSF’ and ‘Chemo + GM-CSF’ denote patients who received myelosuppressive chemotherapy and G-CSF or GM-CSF and had blood cells harvested during recovery from myelosuppression. ‘G-CSF, de novo’ denotes chemotherapy naive patients who received G-CSF at 12 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7. ‘G-CSF + SCF’ denotes chemotherapy naive patient who received G-CSF at 12 μg/kg subcutaneously daily and SCF at 5 to 15 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7 of G-CSF administration. For each group of patients the horizontal bar denotes the median value, the box plot denotes the 5th and the 95th percentiles, and the whiskers denote the maximum and minimum values.
The CFU-GM yield of different mobilization protocols used in the authors' institution. ‘3-4gm Cy’ denotes patients who received cyclophosphamide 3 to 4 g/m2 intravenously and had blood cells harvested during recovery from myelosuppression. ‘7gm Cy’ denotes patients who received cyclophosphamide 7 g/m2 intravenously and had blood cells harvested during recovery from myelosuppression. ‘G-CSF, pretreated’ denotes patients who received G-CSF at 12 μg/kg subcutaneously and had blood cells harvested on days 5, 6, and 7. ‘Chemo + G-CSF’ and ‘Chemo + GM-CSF’ denote patients who received myelosuppressive chemotherapy and G-CSF or GM-CSF and had blood cells harvested during recovery from myelosuppression. ‘G-CSF, de novo’ denotes chemotherapy naive patients who received G-CSF at 12 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7. ‘G-CSF + SCF’ denotes chemotherapy naive patient who received G-CSF at 12 μg/kg subcutaneously daily and SCF at 5 to 15 μg/kg subcutaneously daily and had blood cells harvested on days 5, 6, and 7 of G-CSF administration. For each group of patients the horizontal bar denotes the median value, the box plot denotes the 5th and the 95th percentiles, and the whiskers denote the maximum and minimum values.
TECHNIQUES OF COLLECTION
Timing of Collection
Chemotherapy mobilization.Apheresis usually commences when the leukocyte count rises above 1 × 109/L and continues until an arbitrary target such as 3 × 108 mononuclear cells/kg BW is reached.17 Other groups have used increasing platelet counts or monocytosis as the starting criteria although they usually occur simultaneously. These days CD34+ cell measurement is preferrable because it is a more direct measure of progenitor cells. Most groups use 20 to 40 × 106 CD34+ cells/L as the threshold for starting apheresis.
G-CSF.Apheresis is usually performed on days 5, 6, and 7 of G-CSF administration. Progenitor cell levels start to decrease after day 8 even with continued G-CSF administration, so there is little advantage of continuing apheresis.
Chemotherapy + hematopoietic growth factor.Most groups start apheresis when the leukocyte count is 2 to 5 × 109/L and one report recommends not starting until the leukocyte count is >10 × 109/L.23 CD34+ cell levels should be measured to confirm progenitor cell mobilization.
Single Apheresis
There has been considerable interest in collecting sufficient progenitor cells for rescue from a single apheresis. In our experience, approximately half of previously treated patients undergoing G-CSF or chemotherapy + hematopoietic growth factor (HGF) mobilization achieved >1 × 106 CD34+ cells/kg in a single 2-blood–volume apheresis. However, such a yield can be achieved with a single apheresis in most patients with no previous chemotherapy who received G-CSF or G-CSF + SCF. Several groups are now using large-volume apheresis to maximise progenitor cell yield,174 175 although patient tolerance can be a limitation.
TYPES OF CELLS MOBILIZED
Hematopoietic Progenitor Cells
The number of CD34+ cells transplanted was first proposed by Siena et al42,176 as an indicator of the hematopoietic reconstitutive capacity of blood cells. Following these reports, flow cytometry was widely adopted for enumerating CD34+ cells, although there is no clear consensus as to the most accurate and easily transferable method for this.177 The coexpression of lineage or activation antigens useful for describing progenitor cells within BM178-181 has been used to determine the nature of mobilized CD34+ cells.182,183 Bender et al183 examined chemotherapy-mobilized CD34+ cells to determine the expression of myeloid (CD33) and lymphoid (CD19, CD7) antigens and the transferrin receptor (CD71). They reported that cyclophosphamide-mobilized CD34+ cells were different to BM CD34+ cells in two main respects. Firstly, there were very few pre-B lymphocytes in mobilized blood as determined by the low number of CD34+ cells expressing CD19. Secondly, mobilized CD34+ cells had low levels of CD71 expression, suggesting that they were not actively proliferating. Despite the small number of patient samples analyzed it was clear that variations existed in the incidence of different CD34+ subpopulations in different patients. Another study of chemotherapy + G-CSF mobilized CD34+ cells from 10 patients also showed marked heterogeneity in different patients in the coexpression of CD33 and CD71 antigens.184 The variable, but often high, level of CD71 expression was different than that described by Bender. However, both groups agreed that CD38 and HLA-DR antigens were present on more than 95% of mobilized CD34+ cells. A subsequent study of blood cells elicited by four different protocols indicated that G-CSF administration induced the highest level of CD34+CD38− cells, suggesting that G-CSF–mobilized blood contained a greater proportion of more primitive hematopoietic progenitors than those from other mobilization protocols.39
More recently the presence of putative hematopoietic stem cells, CD34+ Thy-1+ cells in fetal liver, cord blood, and BM has been described.185 In the blood from 35 patients the proportion of CD34+Thy-1+ cells was highest on the first day of apheresis and progressively decreased on each subsequent day of collection.186 A further study examined the proportion of Thy-1+ cells within the CD34+lineage(Lin) negative population and also found that the proportion of CD34+Lin−Thy-1+ cells was dependent on the day of leukapheresis.187 Notably, this study indicated that high levels of CD34+Thy-1+Lin− cells may be present only transiently during mobilisation, despite the continued presence of CD34+ cells. This finding was confirmed by Stewart et al,188 who analyzed the blood and apheresis collections from 25 myeloma patients following cyclophosphamide and GM-CSF–induced mobilization. Although the frequency of CD34+ cells in blood cell collections coexpressing Thy-1 was heterogeneous (ranging from 6.2% to 50% of CD34+ cells), the highest levels were present on the first day of blood cell collection, although the difference in absolute numbers may be less. In a later study, Haas et al189 did not investigate the kinetics of mobilization of CD34+Thy-1+Lin− cells but showed that after G-CSF, the proportion of CD34+Thy-1+ cells in apheresis collections was 1.4-fold more than that in BM obtained on the same day.
These findings indicate that the timing of apheresis may be critical for allogeneic transplantation where the number of CD34+Thy-1+Lin− cells infused may determine long-term hematopoiesis. However, a more recent study by Humeau et al190 highlights the discrepancy in the number of CD34+ cells coexpressing Thy-1 with the number of CD34+CD38− cells, a subpopulation also considered to contain the long-term repopulating cells. They suggested that blood CD34+Thy-1+ cells are not homogenous and contain not only primitive progenitor cells. Although there is now considerable evidence of the importance of the CD34+ cell dose, the value of knowing the dose of CD34+Thy-1+ cells remains uncertain. Moreover, whether the Thy-1+ subset or the CD38− subset provides more accurate information on the long-term hematopoietic reconstitutive capacity of mobilized blood cells still remains to be shown.
Other studies of mobilized blood CD34+ cells have included assessment of CAM expression and proliferative potential.39,40,91,191 To et al39 reported lower CD71 expression and decreased rhodamine 123 retention in mobilized CD34+ cells compared with BM CD34+ cells, suggesting that circulating CD34+ cells are neither actively proliferating nor metabolically active. This characteristic of circulating progenitor cells was also observed in mice mobilized with G-CSF.192 The changes in CAM have been described in an earlier section.
It is important to consider that an immunophenotypically defined subpopulation of mobilized blood cells may not necessarily have the same functional properties as its BM counterpart. To assess the hematopoietic potential of different populations of hematopoietic progenitor cells a number of investigators have performed an extensive range of in vitro assays to document their functional capacity. The CFU-GM assay has long been used to enumerate hematopoietic progenitor cells and to assess the viability of cryopreserved blood cells. However, it does not provide direct information on primitive progenitor cells or progenitor cells committed to other lineages.
There are considerably fewer reports of mobilization of megakaryocytic progenitor cells (CFU-MK and BFU-MK) than of CFU-GM. Increased megakaryocytic progenitors were described in G-CSF–mobilized blood18 and in chemotherapy + hematopoietic growth factor–mobilized blood.193 In a recent study we found an average 75-fold increase in the number of megakaryocytic precursor cells in mobilized blood compared with steady-state blood in 53 patients mobilized by one of six protocols.194 Most of these precursor cells were CFU-megakaryocyte (CFU-Mk), which are likely to represent the progenitor cells responsible for early platelet recovery. There was a high correlation between the number of megakaryocyte progenitor cells and CD34+ cells that confirmed an earlier report showing that the megakaryocytic progenitor cell dose was no better than CFU-GM in predicting platelet recovery posttransplant.195 Hence, it seems that in most situations the number of blood CD34+ cells or CFU-GM infused will provide a guide to platelet reconstitution, at least during the first 2 to 4 weeks posttransplant. However, enumeration of precursor to megakaryocytic progenitor cells may be a better guide to long-term megakaryocytopoiesis.
One significant feature of autologous transplantation with mobilized peripheral blood is rapid lymphocyte reconstitution.48 Such a rapid recovery of T lymphopoiesis could be caused by the very large number of mature T cells or T-lymphoid progenitor cells in mobilized blood cells. The presence of the latter is supported by the demonstration of T-lymphocyte generation in a severe combined immunodeficient-human (SCID-hu) thymus assay by transplanting CD34+Lin− cells isolated from mobilized blood.196
The most primitive hematopoietic progenitor cells assayable in vitro is the long-term culture-initiating cells (LTC-IC). They are present at low levels in steady-state PB197,198 and a fivefold to sixfold higher incidence in mobilized blood.114,199,200 In Sutherland's studies there was more than a 2-log variation in the incidence of LTC-IC between patients and the number of LTC-IC in blood did not correlate with the numbers of CFU-GM, CD34+ cells, or the rate of engraftment. The lack of correlation between LTC-IC and the rate of engraftment is not surprising because the latter probably correlates better with the number of mature progenitor cells infused. The correlation between LTC-IC and long-term hematopoietic reconstitution may be more relevant but remains to be established. The same investigators also claim that LTC-IC in mobilized blood have significantly lower proliferative potential than BM LTC-IC. However, data from another candidate primitive hematopoietic progenitor cells, the precursor to CFU-GM (pre-CFU) as measured in stroma-free cytokine-dependent liquid cultures, suggest that CD34+ cells in mobilized blood have the same proliferative capacity as BM CD34+ cells.36 Nonetheless, both the LTC-IC and the pre-CFU assays are technically demanding and not yet fully quantitative, so are not likely to be used as routine assays in clinical laboratories. Advances in establishing a reproducible and quantitative assay for primitive progenitor cells would provide a much better measure of long-term reconstitutive capacity. Notwithstanding this proviso, the durability of long-term engraftment201 and the presence of primitive hematopoietic progenitor cells in mobilized blood seems beyond doubt. Unequivocal evidence will come from allogeneic transplantation or gene-marking studies.
Accessory Cells
The number of T lymphocytes, monocytes, and natural killer (NK) cells are much higher in PB transplants than BM transplants.48,49,202 These mature accessory cells are probably not critical for hematopoietic reconstitution as evidenced by the satisfactory reconstitution seen in patients rescued with cells positively selected for CD34+ cells. However, autotransplants using combined BM and mobilized blood cells containing only a low number of CFU-GM suggested that hematopoietic reconstitution occurred earlier than if BM alone or BM plus unmobilized blood cells were used.6,203 Furthermore, mononuclear cells obtained during hematopoietic recovery secrete more cytokines than those harvested during steady-state hemopoiesis.204 A study of cytokine levels posttransplantation also suggests that IL-6, G-CSF, and IL-8 levels are related to neutrophil recovery after stem cell transplantation. It is particularly noteworthy that the levels of IL-6, G-CSF, and IL-8 are higher following blood cell transplants than BM transplants.205 Because mononuclear cells are a known source of such cytokines it seems reasonable to attribute a complimentary role of accessory cells to hematopoietic reconstitution.
The immune reactivity of donor blood leukocytes in the control of leukemia after allogeneic BM transplants has been well documented.206 In autologous transplantation the high levels of NK cell numbers and activity in mobilized blood cells raise the possibility of using blood cells as a cell source for immunotherapy as well as hematopoietic reconstitution. In GM-CSF–mobilized blood cells the frequency of cytotoxic effector cells including lymphokine-activated killer (LAK) cells and lymphocytes was elevated, especially in the early harvests, whereas the later harvests contained more progenitor cells49; however, the difference in absolute numbers may be less. In cyclophosphamide + G-CSF–mobilized blood cells functionally active NK cells were present at 42 to 212 × 106/kg in the grafts and their percentage and cytotoxic activity increased from the beginning to the end of the harvesting procedure. CD3−CD56+ and CD34+ cell numbers peaked at the same time. A 6 to 8 days' incubation with 100 U/mL, IL-2 expanded the NK population threefold to fivefold without adversely affecting CD34+ cells.202 Hence, the immunomodulatory potential of mobilized blood cells is worthy of further investigation.
Standardization of Progenitor Cell Measurement
CFU-GM and CD34+ cell measurements are the two most commonly used indices of the hematopoietic reconstitutive capacity of transplanted cells, but the standardization of these measurements remains elusive. Firstly, methodology in different laboratories varies greatly. Personal preferences and established practices all contribute but the large number of biological reagents involved and the lack of a ‘standard’ pose significant hurdles. Secondly, even in a single laboratory, the type of colony stimulatory factor, the batch of fetal calf serum, and the training of staff influence CFU-GM results significantly.207 For instance, a four-factor combination of G-CSF, GM-CSF, IL-6, and SCF stimulates 80% more CFU-GM than human placental conditioned medium although costing 20 times more. New staff members also show more variance in their colony counting than experienced members.
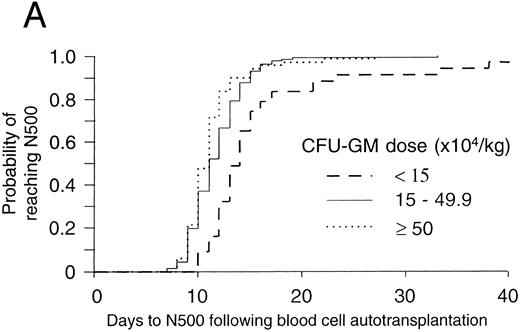
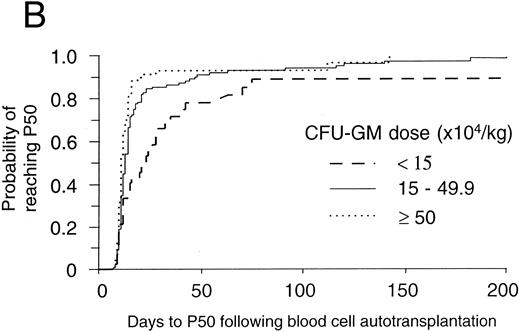
The threshold effect of progenitor cell dose on hematopoietic reconstitution after mobilized blood cell autotransplants in the authors' institution. In (A) and (B), patients were categorized into those receiving <15, 15 to 49.9, and ≥50 × 104 CFU-GM/kg BW. In (A) the probability of reaching 0.5 × 109 neutrophils/L (N500) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The number of patients in the three categories were 47, 142, and 88, respectively. The differences between the three categories were significant (P < .0001, log-rank test). In (B) the probability of reaching 50 × 109 platelets/L (P50) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The number of patients in the three categories were 45, 131, and 86, respectively. The differences between the three categories were significant (P < .0001, log-rank test).
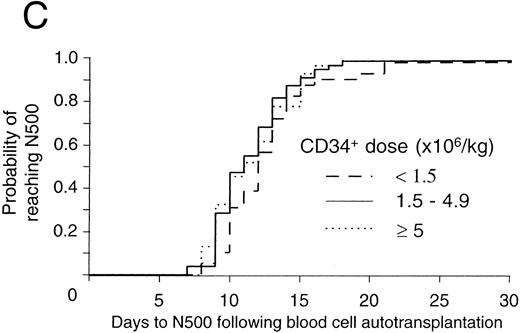
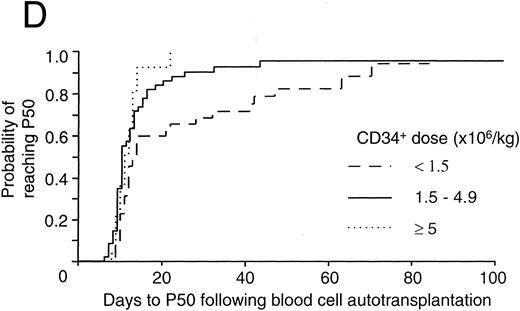
In (C) and (D) patients were categorized into those receiving <1.5, 1.5 to 4.9, and ≥5 × 106 CD34+ cells/kg BW. The number of patients in these three categories were 39, 53, and 31, respectively. In (C) the probability of reaching 0.5 × 109 neutrophils/L (N500) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The differences between the three categories were not significant (P = .29, log-rank test). In (D) the probability of reaching 50 × 109 platelets/L (P50) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The number of patients in these three categories were 35, 49, and 27, respectively. The differences between the three categories were significant (P = .0052, log-rank test).
The threshold effect of progenitor cell dose on hematopoietic reconstitution after mobilized blood cell autotransplants in the authors' institution. In (A) and (B), patients were categorized into those receiving <15, 15 to 49.9, and ≥50 × 104 CFU-GM/kg BW. In (A) the probability of reaching 0.5 × 109 neutrophils/L (N500) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The number of patients in the three categories were 47, 142, and 88, respectively. The differences between the three categories were significant (P < .0001, log-rank test). In (B) the probability of reaching 50 × 109 platelets/L (P50) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The number of patients in the three categories were 45, 131, and 86, respectively. The differences between the three categories were significant (P < .0001, log-rank test).
In (C) and (D) patients were categorized into those receiving <1.5, 1.5 to 4.9, and ≥5 × 106 CD34+ cells/kg BW. The number of patients in these three categories were 39, 53, and 31, respectively. In (C) the probability of reaching 0.5 × 109 neutrophils/L (N500) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The differences between the three categories were not significant (P = .29, log-rank test). In (D) the probability of reaching 50 × 109 platelets/L (P50) for each of these three categories was denoted by the lines - - -, — , ⋅⋅⋅, respectively. The number of patients in these three categories were 35, 49, and 27, respectively. The differences between the three categories were significant (P = .0052, log-rank test).
There was initial optimism that CD34+ cell enumeration may be preferable to clonogeneic assays as a measure of hematopoietic progenitor cells because fewer biologicals are required and electronic counting of large number of events should have a lower coefficient of variance. However, CD34+ cell enumeration protocols need to achieve the level of stringency to accurately measure rare events at the level of 0.1% to 3%. While a reliable, accurate, and transferable method for CD34+ cell enumeration is the aim of multiple intergroup collaborations, there is no agreement on a method of enumeration by multiparametric flow cytometry.42,182 208-212
Several issues are critical in achieving standardization in CD34+ cell enumeration. Firstly, the method of sample processing influences the degree of cell debris and red blood cell contamination. Secondly, the CD34 antibody and the fluorochrome label used influence the specificity and sensitivity of immunolabeling. Thirdly, the gating strategy, including the denominator for the number of CD34+ cells, is critical in establishing the incidence of CD34+ cells relative to other leukocytes. It is essential that individual laboratories have well-documented methods, an appreciation of the statistical variance of their method and systems for internal and external comparisons with other laboratories. Laboratories should also consider adopting one of the standardized methods being developed by agencies such as the International Society for Hematotherapy and Graft Engineering.213 For a comprehensive review of this topic readers should consult the June 1996 issue of the Journal of Hematotherapy.213-216
The reporting of a reference range based on healthy individuals would provide a means of establishing comparability of data between different laboratories using different methods for enumerating CFU-GM or CD34+ cells. Such a practice is mandatory in most laboratory measurements and it is surprising that it has gained little support among transplantation laboratories.
TARGET AND THRESHOLDS
A target progenitor cell yield from leukaphereis is important because the number of progenitor cells infused correlates with the rate of hematopoietic reconstitution which affects the safety and costs of transplants. The target yield then determines when and how many leukaphereses are required. Unlike BM transplants, nucleated cell dose does not appear to be as effective as progenitor cell dose in predicting hematopoietic reconstitution. Furthermore, the progenitor cell dose:hematopoietic reconstitution relationship in blood cell transplants can be described in terms of a minimum and an optimum threshold for rapid reconstitution, a threshold for sustained reconstitution, and a threshold of rapid reconstitution which cannot be lowered even with very high progenitor cell dose and cytokine administration.41,42,44,45,76,116,160 217-219 A highly significant correlation between CFU-GM and CD34+ cells as indices of hematopoietic reconstitutive capacity has been confirmed by numerous reports so a threshold based on either assay is equally valid.
A CFU-GM cell dose effect was first suggested in 198641 when analysis of the limited data available then suggested a minimum of 30 to 50 × 104/kg BW for rapid hematopoietic reconstitution. In G-CSF–mobilized blood cell transplants Sheridan et al220 reported that patients receiving >30 × 104 CFU-GM/kg BW recovered faster than those receiving less. In another report patients receiving <20 × 104 CFU-GM/kg had a significantly lower probability recovering to 0.5 × 109 neutrophils/L by day 13 and 50 × 109 platelets/L by day 15 compared with those patients receiving the above-mentioned threshold progenitor cell dose.45 Currently 15 to 20 × 104 CFU-GM/kg or 1 to 2 × 106 CD34+ cells/kg is generally agreed as the minimum threshold below which rapid hematopoietic reconstitution may not occur.44 76
A progenitor cell dose above the minimum threshold is associated with increasingly rapid hematopoietic reconstitution.76,221 However, there seems to be an upper threshold effect at 50 × 104 CFU-GM/kg or 5 to 8 × 106 CD34+ cells/kg, above which further increase in cell dose does not further hasten recovery.42,44,46,116 Bender et al44 also suggested that there may be a minimum threshold of 5 to 20 × 104 CFU-GM/kg or 0.5 to 2 × 106 CD34+ cells/kg for long-term reconstitution.
Figure 3 shows the different probabilities of neutrophil and platelet reconstitution in patients receiving different numbers of progenitor cells in our institution. Those receiving less than the minimum threshold numbers have a significantly higher probability of slow reconstitution while those receiving above the optimum threshold number have a 90% or higher probability of rapid reconstitution. However, patients receiving >8 × 106 CD34+ cells/kg BW do not recover more rapidly than those receiving 5 to 8 × 106/kg BW. The same trend of minimum and optimum threshold is seen across the spectrum in patients with different diseases, mobilization protocols, and high-dose therapy.
In patients with acute myeloid leukemia (AML) transplanted with mobilized blood cells a secondary decrease in platelet counts often occurred during the second month posttransplant after initial rapid reconstitution. Even in patients administered ≥100 × 104 CFU-GM/kg the platelet count decreased to below 20 × 109/L although eventual recovery usually occurred.218 Such a pattern is not seen in non-AML patients and there is no lack of megakaryocytic progenitors in the mobilized blood cells of these patients.222 Hence, a minimum threshold for sustained platelet reconstitution has not been identified in AML patients.
Previous chemotherapy is another variable considered important in determining progenitor cell threshold for rapid engraftment. Tricot et al160 analyzed 225 patients receiving autologous mobilized PB transplants for multiple myeloma and found that the minimum CD34+ cell threshold was ≥2 × 106/kg for patients with ≤24 months of chemotherapy but ≥5 × 106/kg for patients with longer chemotherapy exposures. Whether this reflects a poorer quality of CD34+ cells after prolonged chemotherapy or concomitant stromal damage interfering with engraftment remains to be determined.
Ex Vivo Expansion of Progenitor and Postprogenitor Cells
An obligatory delay of 7 to 10 days before clinically significant engraftment persisted even when large numbers of progenitor cells, eg, 400 × 104 CFU-GM/kg, were re-infused. Even in patients who received mobilized blood cells as well as G-CSF posttransplant the median time to neutrophil and platelet engraftment is little better than mobilized blood cells alone.20,223 This time delay is similar to the time for colony formation in vitro and thus probably reflects the time required for infused progenitor cells to home to BM and then to divide and mature to provide detectable engraftment.36 45
This observation leads to studies of ex vivo expansion of progenitor and postprogenitor cells with the aim of abrogating cytopenia posttransplant. Cell proliferation and orderly differentiation stimulated by myeloid growth factors and/or hematopoietic stroma have been shown both in small- and large-scale cultures36,224-227 with the hematopoietic growth factor combination and the culture system used identified as important variables. The hypothesis is that infusion of promyelocytes and myelocytes produced ex vivo from mobilized CD34+ cells may produce earlier engraftment.36 In theory, appropriate culture systems may also expand erythroid and megakaryocytic cells.
The lack of toxicity of such ex vivo–expanded cells has been demonstrated with up to 157 × 106 cells/kg infused,228 although no reduction in cytopenia was evident. However, PIXY321 was the only hematopoietic growth factor used in this study so cells expanded using more active combinations such as G-CSF, GM-CSF, IL-3, or IL-6 may be more effective.36 225
Ex vivo expansion has also been applied to expanding a small aliquot of mobilized cells to provide sufficient progenitor cells for hematopoietic rescue.229 This will reduce the need for leukapheresis and possibly the number of contaminating malignant cells infused. Another important potential application of ex vivo expansion is in expanding an apheresis product with a low number of progenitor cells to provide sufficient cells for rescue. The feasibility of such an approach is not known.
Difficult to Mobilize Patients
In most reports there is a subset of patients, most of them heavily pretreated, who did not achieve satisfactory progenitor cell mobilization. Inherent biologic factors do exist as demonstrated by poor mobilization in SCF– or c-kit–deficient mice compared with wild-type mice103 and the considerable heterogeneity in progenitor cell yield even in patients with no previous chemotherapy receiving G-CSF.123 No generally agreed strategy exists for patients who failed to mobilize with G-CSF or chemotherapy + hematopoietic growth factors. The IL-3–GM-CSF combination was not particularly effective, although combinations of G-CSF and SCF, G-CSF, and Flt3 ligand hold promise. Direct mobilizers such as IL-8 alone or in combination with other cytokines may be more effective. Better understanding of the mechanism of mobilization may also contribute to the development of better mobilization protocols for difficult-to-mobilize patients.
CLINICAL RESULTS
Hematopoietic and Immune Reconstitution
There is now overwhelming evidence that trilineage recovery occurs within 2 weeks of transplant using mobilized blood cells provided the above threshold numbers of progenitor cells are given. Initial studies were mostly phase I/II studies based on comparison with historical or concurrent but nonrandomized BM transplant patients. However, reports of randomized studies are now appearing. A randomized comparison of 46 patients with relapsed or refractory germ cell tumors randomized to either BM or G-CSF + chemotherapy mobilized blood cell rescue showed that mobilized blood cells result in quicker hematopoietic reconstitution, leading to fewer days on intravenous antibiotics and a shorter time to transfusion independence.230
On first principles, subsets of CD34+ cells may provide more specific information on lineage recovery. However, neutrophil progenitors as measured by CD33+CD34+ cells have not been shown to be more informative than CD34+ cells. This may be due to the difficulty in defining CD33 positivity because CD33 expression in CD34+ cells tends to be more like a continuous population than distinct positive/negative populations.39 The measurement of megakaryocytic progenitor cell in PB based on CD41 or CD61 expression has been bedevilled by high false-positivity secondary to platelet binding to monocytes.231 In contrast, Derskson et al97 reported that the CD34+L-selectin+ cell dose predicts rapid platelet recovery after blood cell transplants using chemotherapy + G-CSF mobilization. How the expression of an adhesion molecule influences platelet reconstitution is not yet known, although the investigators suggested that enhanced homing may be responsible.
In allogeneic and autologous BM transplants the predominant change in immune status is suppression. However, the transplant of circulating, long-living, immune competent cells was first described in 1972 for the treatment of chronic mucocutaneous candidiasis with leukocytes from HLA-compatible siblings.68 In 1979 lymphoid reconstitution after blood cell transplant was shown to be more rapid than that following BM transplants in animal studies.232 In humans rapid T-lymphocyte recovery after autologous blood cell transplant was first described in 198747 and confirmed by several other studies.48,233,234 In a comparative study of 49 blood cell transplants and 18 BM transplants, Roberts et al48 reported that recovery of lymphocyte count, CD3, CD4, and CD8 cells was significantly faster after blood cell transplants than allogeneic BM transplants. CD8+ cells recovered within 2 weeks posttransplant whereas CD4+ cells lagged behind with an inversion of the CD4/CD8 ratio. Although such a pattern is similar to that in BM transplants, CD4 recovery to 0.2 × 109 cells/L occurred faster in blood cell transplants than in BM transplants. This CD4 threshold has been identified as a level below which significant infective risk occurs in human immunodeficiency virus (HIV) patients.235 Donor T-cell clones have shown ability to transfer cellular immunity against cytomegalovirus in allogeneic BM transplants,236 so it is possible that infused T cells in blood cell transplants contribute to reconstitution of immunity and therefore lower antibiotic usage.
Roberts et al48 reported that CD20 cells were significantly higher after blood cell transplants than allogeneic BM transplants, but recovery of NK cells showed no difference between transplant types. Functional NK cell recovery paralleled numerical recovery with rapid reconstitution 10 to 14 days after transplantation237 and full recovery after 4 to 5 weeks.202 IL-2 after autologous BM transplants in acute leukemia has been suggested as a means of reducing relapse. The rapid NK recovery in blood cell transplants may provide an even better setting to test this hypothesis.
Before We All Say Blood, ...
Since the change from using BM to mobilized blood cells requires re-equipping (blood cell separator, flow cytometry), retraining (apheresis staff ), and sometimes rescheduling (optimally timed blood cell harvesting requires rapid deployment of apheresis and laboratory staff ), it was often asked whether one can obtain sufficient cells from BM. A Danish group has studied whether cytokine priming of BM before harvest would lead to a more rapid hematopoietic reconstitution without the need for leukapheresis.238 In their latest report Johnsen et al239 described patients receiving either IL-3 10 μg/kg/d for 10 days, GM-CSF 10 μg/kg/d for 5 days, or G-CSF 10 μg/kg/d for 5 days. Cytokine priming led to a twofold to fourfold increase in mean light-density cell and CFU-GM yield in a standard 1-L BM harvest. However, in the 17 patients who received primed BM (4 receiving IL-3, 5 receiving GM-CSF, and 8 receiving G-CSF ), the rate of hematopoietic reconstitution was no faster than historical controls receiving unprimed BM but 10 to 20 days slower than a parallel cohort of patients receiving G-CSF–mobilized blood cells.239
Another recent report described no difference in neutrophil and platelet recovery between G-CSF–mobilized blood cell and G-CSF–primed BM autotransplants.240 Because G-CSF was used posttransplant, any difference in neutrophil reconstitution due to the type of cells used had been minimized.17 223 Why platelet reconstitution was no different between the two groups is intriguing, although this may be partly explained by the presence of activated accessory cells and G-CSF–primed blood cells in the BM used.
Nonetheless, this issue of BM or PB should be re-examined whenever new cytokines or improved scheduling or combinations of cytokines become available. It is conceivable that a combination of G-CSF, thrombopoietin, and early acting factors such as SCF or Flt3 ligand may stimulate BM sufficiently to provide high numbers of progenitor cells for rapid engraftment and multiple rescues.241
Tumor Control
Since the benefit of mobilized blood cell transplant is shorter hospitalization, lower blood products and antibiotic usage, and lower procedure-related mortality, such transplants should not by themselves lead to a different tumor control outcome than BM transplants.242 However, the increased safety and high number of progenitor cells harvested open up additional options.
Older patients and more diseases are now treated with blood cell transplants compared with BM transplants. It is now commonplace to perform transplants in patients above 60 and most transplants in breast cancer are performed with mobilized blood cells.
An even more important development is the use of multiple cycles of high-dose chemotherapy and blood cell transplants to deliver a higher total dose and dose rate.123 243-245 In many instances, myelosuppressive rather than supralethal doses were administered, but the emphasis is rightly that of tumor cell kill rather than myeloablation. This applies particularly to the more slowly growing tumors because multiple submaximal high-dose therapy may achieve a higher cumulative cell kill than a single intensive assault.
Bezwoda et al246 described a randomized study comparing double high-dose therapy (cyclophosphamide 2.4 g/m2, mitoxantrone 35 to 45 mg/m2, and etoposide 2.5 g/m2 6 weeks apart) and mobilized blood cell rescue with 6 to 8 cycles of conventional-dose cyclophosphamide 0.6 g/m2, mitoxantrone 12 mg/m2, and vincristine 1.4 mg/m2 as first-line therapy for metastatic breast cancer in 90 patients. The high-dose therapy group had a significantly higher response rate (95% compared with [c.f.] 53%) and complete remission rate (51% c.f. 4%), and longer median duration of response (80 weeks c.f. 34 weeks) and survival (90 weeks c.f. 45 weeks). The prolongation of response and survival times was almost entirely due to the relative proportion of patients who achieved complete remission, which was most probably caused by the dose escalation. Such improvements are clinically noteworthy irrespective of whether eventual cure occurs.
Other issues that may impact on tumor control include CD34+ cell selection, tumor purging, and the effect of accessory cells. A 3 to 5-log reduction in malignant contamination in the transfused cells is achievable by CD34+ cell selection.38,247 However, the presence of the clonotypic BCL2-IgH rearrangement in CD34+CD19+ progenitor cells in patients with follicular lymphoma raises the question that selection based on CD34+ expression alone may be insufficient.248 Tumor-directed purging may be more specific but its application is limited to AML, neuroblastoma, and lymphoma because a specific tumor marker is required. The large number of accessory cells may be targeted for immunomodulation to enhance ‘graft-versus-tumor’ effect, although no data are available.
A number of such innovations was incorporated in a report of 21 patients with non-Hodgkin's lymphoma who received hematopoietic rescue using mobilized blood cells collected in a single apheresis product which was enriched for CD34+ cells by a discontinuous Percoll gradient (Pharmacia, Uppsala, Sweden) and then purged with a panel of anti-B cell or anti-T cell monoclonal antibodies and complement.249 In the authors' institution a current study in myeloma patients involves 2 cycles of high-dose therapy rescued with immunomagnetically selected CD34+ cells from mobilized blood but the second selection is performed on cells harvested during recovery from the first high-dose therapy. Such an approach combines multiple-cycle chemotherapy with in vitro and in vivo purging. With the large number of CD34+ cells and accessory cells available in mobilized blood, multiple modality approaches can be tested.
Malignant Contamination
The improvement in the mobilization of blood progenitor cells and the increasing ability to deliver dose-intensified therapy has not been matched by an advance in our understanding of the mobilization of malignant cells. Recent advances in the detection of low levels of tumor cells/markers have provided much-needed information on malignant contamination in autografts.
Acute leukemia.Small numbers of leukemic cells among normal hematopoietic progenitors can be distinguished using morphologic and cytochemical examinations, but they are both laborious and insensitive. Better techniques are based on the identification of karyotype abnormalities by in situ hybridization, immunophenotypic abnormality by flow cytometry, genetic abnormalities and antigen-receptor gene rearrangements by polymerase chain reaction (PCR), and abnormal cell growth by clonogenic assays.250
We have recently performed serial trisomy 8 studies using a centromeric probe for fluorescent in situ hybridisation (FISH) in an AML patient whose leukemic cells bear a trisomy 8 abnormality (White D, et al, submitted). Although all remission (postinduction and posttransplant) BM and PB metaphases showed a normal karyotype, FISH studies consistently detected a level of 3% to 12% trisomy 8 in interphase cells. The level in chemotherapy-mobilized blood cells was similar to steady-state BM and PB. Combined flow cytometric sorting and FISH analysis showed that the trisomy was not present in lymphoid cells so the persistent trisomy postinduction and transplant was not caused by long-living lymphocytes. The patient relapsed 3 months after an autologous transplantation using remission BM and blood cells in first remission. Hence, leukemic cells appear to be present even during clinical and cytogenetic remission. Together with the genetic marking studies251 it seems that leukemic cells are present in BM and mobilized and steady-state blood cells in some cases of AML and they may contribute to relapse. This is consistent with reports showing the advantage of purging in autologous transplantation in AML.252 Nonetheless, not all patients receiving autologous transplants relapse,253 so whether leukemic contamination occurs in all patients is not yet known.
In acute lymphoid leukemia (ALL) persistence of the leukemia-associated molecular marker indicates a higher risk of relapse,254,255 although not all PCR-positive patients had relapsed. Seriu et al255 reported that 8 of 13 mobilized leukapheresis products from 5 children were PCR-positive, although the levels were generally lower in PB than in BM. One patient transplanted with PCR-negative blood cells relapsed first in the central nervous system and then in BM. Three of four children transplanted with PCR-positive blood cells relapsed within 6 months, and yet one remained in remission 17 months posttransplant. Hence, mobilization of leukemic cells seems to occur in ALL and transplant with leukemic marker–positive blood cells in ALL appears to be associated with a high risk of relapse.
Lymphoma and multiple myeloma.Sharp et al256 studied BM and blood cell harvest specimens and reported the proliferation of cells carrying the same Ig gene rearrangement as the original lymphoma. Other groups have also demonstrated lymphoma-associated molecular marker in mobilized blood cells although no quantitative comparison with BM or steady-state blood was made.30 257
Ig gene fingerprinting has also enabled the detection of myeloma cells in mobilized blood cells. A monoclonal population was detected in 44% of 22 patients and their presence was correlated with serum β-2-microglobulin levels. It was suggested that blood cell contamination defines poor-risk patients.258 Henry et al259 described PCR-positive harvests in 7 of 8 multiple myeloma patients but estimated that the number of myeloma cells infused would be lower with blood cell than with BM transplants.
Breast and ovarian cancer.Sharp et al256 also described the identification of morphologic malignant breast cancer cells in BM from breast cancer patients. They further reported that those with positive culture did worse after autologous transplantation than those without. The detection of breast cancer cells in BM and mobilized blood cells has since been reported by several groups.30,260 Brugger et al30 showed that cytokeratin (CK)- or epithelial antigen (HEA)-positive cells measured immunocytochemically were present in PB of 29% of patients with stage IV breast cancer. After chemotherapy + G-CSF, 21% of those without demonstrable circulating CK/HEA+ cells during steady state became positive. All stage IV breast cancer patients tested positive. Interestingly the timing of CK/HEA+ cell mobilization seems to depend on BM involvement. In patients without BM involvement, CK/HEA+ cell mobilization occurred in the first week after chemotherapy. In patients with BM involvement CK/HEA+ cell mobilization occurred in the second week, at the same time as CD34+ cell mobilization. These findings, if confirmed, may have major impact on progenitor cell mobilization in patients with stage IV breast cancer. However, the specificity of the HEA antibodies has been questioned and the tumorigenicity of these cells is yet to be determined.
A soft-agar culture stimulated by GM-CSF, epidermal growth factor, and insulinlike growth factor to detect viable breast cancer cells was pioneered by Ross et al28 and has the potential advantage of demonstrating viability. Tumor cell detection by this method correlated with the immunocytochemical method. Malignant contamination in mobilized blood cells appeared to be less frequent than BM and may even be absent.28,29 261 No data on the time course of mobilization were reported.
In an immunocytochemical study of epithelial contamination in mobilized blood cells and BM from 22 patients with ovarian cancer, mobilized blood cells had a lower tumor contamination than BM.29
The presence of ‘tumor’ cells by morphologic examination, the demonstration of CK+ cells, or the detection of a clonal genetic mutation by molecular or cytogenetic techniques are all important findings because they suggest strongly that cells of the neoplastic lineage are present. The growth of clonogenic tumor cells provides an even stronger evidence. Hence, malignant contamination of mobilized blood cells does occur, although probably less than with BM.
The crucial question of whether cells or molecular markers indicative of the target neoplasm represent tumor stem cells or effete and cells is less easy to answer. Their association with relapse may indicate a persistent though subclinical residual tumor rather than causative effect. The notable exception is in AML and neuroblastoma where gene-marking studies have shown that the autologous cells infused contributed to relapse.251 Even there the conclusion still falls short of proving that they cause relapse. Moreover, several salient clinical observations remind us that not all neoplastic cells cause relapse.
Neiderwieser et al262 described how a female patient with chronic myeloid leukemia achieved complete remission after receiving BM from her brother donor, who unfortunately developed acute leukemia himself. Even though the transplanted BM contained 27% myeloblast, engraftment occurred with normal but not leukemic male cells. She relapsed with her brother's leukemic cells 6 months later. The initial engraftment with normal donor cells instead of donor leukemic cells suggests that most leukemic cells do not cause relapse and that only the leukemic stem cells can.
Goldman et al10 reported 20 patients with Philadelphia chromosome (Ph′ )-positive chronic myeloid leukemia (CML) who received autologous chronic phase blood cell transplant. Although a chronic phase was restored in most patients, 2 patients achieved Ph′-negative hematopoiesis after a period of cytopenia.10 263 Hence, the infused chronic phase Ph′-positive blood cells failed to engraft even though there was no reason why they should be rejected.
These observations compel us to examine the question of malignant contamination more critically. Whether the tumor stem cell exhibits the same phenotype as the majority of tumor cells is unknown. Furthermore, whether all viable cells that bear the same immunophenotype and molecular phenotype are capable of metastasis is yet to be determined. The SCID mouse/leukemic cell xenograft model is already providing insights into the different immunophenotype of leukemic stem cells and their progeny,264 and may provide a more relevant assay of the significance of such cells. At a more basic level it would also be important to identify whether tumor cells are mobilized by the same mechanism(s) as CD34+ cells and to explore whether differential mobilization of normal cells can be achieved.
SPECIAL AREAS
Cost Effectiveness Analysis
Chao et al122 compared the short-term medical costs between patients with relapsed Hodgkin's disease transplanted with steady-state blood and patients transplanted with G-CSF–mobilized blood cells, both groups receiving G-CSF posttransplant. There was an average 45% net decrease in costs associated with the use of G-CSF–mobilised blood cells, mainly due to earlier platelet recovery and shorter hospitalization. Another retrospective comparison between the costs of mobilized blood cell and BM transplants in France showed that blood cell transplants, at a mean of $29,000, cost $7,800 less.265
A recent report compared the survival, quality of life, therapy costs, and cost-effectiveness in three groups of myeloma patients: Durie-Salmon classification grade III receiving high-dose therapy and melphalan-mobilized blood cell rescue (group I); grade III receiving conventional polychemotherapy (group II); and grade II receiving conventional chemotherapy (group III).266 The median survival and quality of life index (based on a 10-step scale) were significantly lower in group II than in group I. The quality of life index of group III was also lower than group I although survival was not. The total global cost based on the mean duration of and degree of dependency during hospitalization, mean numbers of laboratory analysis, radiologic investigations, and operative procedures and outpatient care costs were higher in group I than the other two groups (US $56,700, $46,555, and $37,430, respectively). However, for absolute cost-effectiveness (corrected for survival) the costs per week of life gained of group I (US $350) compared extremely well with those of group II (US $1,862). Furthermore, qualitative cost-effectiveness (weeks of survival gained, corrected for quality of life) was only +US $74/wk comparing groups I and II.
Although the quality-of-life scale is relatively crude and the therapy cost models are retrospective estimates, these reports nonetheless represent a praiseworthy effort in addressing the cost-benefit issues of blood cell transplantation. There is a cost saving associated with the change over from BM to blood cell transplants due to lower resource utilization. Blood cell transplants may be more expensive than conventional chemotherapy in the short term but would most probably lead to an improved cost-effectiveness when there is a significant difference in response and progression-free survival. The critical question of what is the cut-off point for medical costs versus quality and quantity of life gained will be different for different treatment protocols and diseases. To insist on such measurements to evaluate all new treatment modalities will become a more urgent and unavoidable call.
Special Issues in Allogeneic Blood Cell Transplantation
Allogeneic hematopoietic progenitor cells provide a tumor-free graft with proven or potential graft-versus-tumor activity. In aplastic anemia, acute leukemias, CML, myelodysplasia, and genetic diseases, allogeneic cells have been the cell of choice. In the first report of allogeneic blood cell transplantation, the patient received nonmobilized blood cells but died too soon posttransplant to be evaluable.267 More recently, numerous reports of successful transplants using G-CSF–mobilized blood cells from allogeneic donors have appeared.124-128 Rapid hematopoietic reconstitution, no increased graft-versus-host disease (GVHD), and donor preference encourage the use of mobilized blood cells instead of BM in the allogeneic setting as in the autologous setting.
There was initial caution about allogeneic blood cell transplantation because of the potential of severe GVHD from the high number of T cells infused and the uncertainty about the safety of administering recombinant growth factor for mobilization. The accumulated experience has dispelled the fear of severe GVHD and demonstrated the short-term safety of administering G-CSF to donors even though the long-term effect is still unknown. Many donors and investigators consider that the avoidance of general anesthesia, soft tissue, and bone trauma more than compensates for the potential risk of G-CSF administration. These issues have been addressed in a recent annotation.50
Most transplants were performed using unmodified blood cells so the number of T cells infused ranges from 1 to 5 × 108/kg BW compared with <0.5 × 108/kg BW in BM transplants. The lack of severe GVHD suggests strongly that its occurrence relates more to genetic incompatibility than to the number of T cells infused. An alternative hypothesis is that T cells in mobilized blood cells are different from those in BM. Further studies are required to elucidate this observation. Whether CD34+ selected blood cells offer any advantage is not known, but the lower number of T cells infused may enable less intensive GVHD prophylaxis.
Hematopoietic reconstitution in the initial reports was not as fast as that in autologous mobilized blood cell transplants. This is probably due to the use of methotrexate in GVHD prophylaxis because the rapidity of reconstitution in patients receiving cyclosporin and prednisolone is more comparable.268 Sustained long-term engraftment beyond 2 years has been shown124,128 with no report of late graft failure. Naive and memory helper T-cell and B-cell recovery is more rapid after mobilized blood cell transplants than BM transplants.269 Proliferative responses to phytohemagglutinin, pokeweed mitogen, tetanus toxoid, and candida were also higher.269 In a patient with mucopolysaccharidase deficiency type VI transplanted with G-CSF–mobilized allogeneic blood cells, serum galactosamine-4-sulfatase level increased from 0% to 100% day 9 posttransplant at the same time when hematopoietic reconstitution occurred (Toogood I., unpublished data). Hence, functional reconstitution of enzyme activity seems equally rapid.
The large number of progenitor cells in mobilized blood cells may also provide additional leverage to overcome rejection associated with hematopoietic cell transplantation across major HLA barriers.270 This was borne out in patients transplanted with T-cell–depleted BM and mobilized blood cells from haplo-identical family members.271 They showed rapid and sustained hematopoietic reconstitution without significant GVHD. The potential impact of using a high progenitor cell dose to force engraftment even in mismatched or unrelated transplants is enormous.
The EBMT consensus statement on allogeneic blood cell transplantation provides a set of state-of-the-art recommendations.50 They include G-CSF at 10 μg/kg/d, starting leukapheresis on the day after the fourth dose with a continuous flow blood cell separator using peripheral veins, processing up to 15 L of blood per day, CD34+ cell target of 2 to 3 × 106/kg, and standard GVHD prophylaxis. The critical question of whether allogeneic blood cells may replace BM may be answered in the next few years.
CML and Myelodysplasia
The predominance of the Ph′-positive, BCR-ABL rearranged clone in CML is well established. However, the presence of ‘normal’ stem cells in chronic phase has also been shown.272,273 While allogeneic and matched unrelated transplantation have been the preferred treatment, autologous transplants have been explored in patients without a histocompatible donor. A most intriguing observation was the establishment of a Ph′-negative remission after autologous transplant with chronic-phase blood cells.10,263 Furthermore Korbling et al9 showed that Ph′-negative CFU-GM could be detected during the recovery phase following chemotherapy similar to that used for AML. This phenomenon was exploited by Carella et al274 using a combination of G-CSF and intensive induction chemotherapy. Ph′-negative remission has been achieved after autologous transplantation using mobilized blood cells. Ex vivo selection based on CD34 and HLA-DR expression,275 functional isolation of primitive stem cells,276 long-term culture,277 ribozymes,278 and anti-sense oliginucleotides279 are strategies that may deplete the Ph′-positive clone in mobilized blood cells. Hence, autologous transplant is a therapeutic option to be considered in patients not eligible for allogeneic transplants.
Autologous transplant is usually not recommended for myelodysplasia because of doubts about how frequent residual normal cells are in this condition. However, the mobilization of polyclonal primitive hematopoietic progenitor cells in patients with high-risk myelodysplasia after induction chemotherapy has been reported recently.280 This is analogous to CML and may provide a new option for patients without a histocompatible donor.
Pediatric Patients
The most comprehensive series of reports of pediatric blood cell harvesting and transplants comes from Takaue et al.281 The main distinctives in this pediatric population are special requirements for vascular access and leukapheresis, high progenitor yields, and risks of ‘stem cell exhaustion.’ Sustained cytopenia after blood progenitor cell collection occurred in two 3-year-old children with cancer, although adequate hematopoietic reconstitution was re-established after high-dose therapy and stem cell rescue.282 The investigators suggested that the mobilizable pool is a major component of the total body pool in small children during hematopoietic recovery.
Applications in Nonmalignant Diseases
Paroxysmal nocturnal hemoglobinuria (PNH) is associated with a deficiency of decay accelerating factor (DAF ) and membrane inhibitor of reactive lysis (CD59) on hematopoietic cells. Prince et al283 recently reported that CD34+CD38− cells in G-CSF– or GM-CSF–mobilised blood are relatively enriched for DAF+CD59+ cells compared with those in steady-state blood and suggested that apheresis sample can serve as a source of unaffected stem cells for autologous transplantation of PNH patients.
High-dose immunosuppressive therapy such as total nodal irradiation, antilymphocyte globulin, and high-dose cyclophosphamide have been suggested for the treatment of multiple sclerosis.284 However, experimental studies suggest that total lymphoid and myeloid ablation and subsequent recapitulation of lymphocyte ontogeny by hematopoietic rescue may stop the auto-immune destruction of myelin. Although radical at first sight, this type of approach has already been applied to aplastic anemia, a probable auto-immune disease. There are also anecdotal clinical observations that patients with a coincidental auto-immune disease such as psoriasis, ulcerative colitis, and rheumatoid arthritis who undergo BM transplants for other reasons have durable remissions of their autoimmune disease. Hence, autologous transplant has been proposed for the treatment of severe autoimmune disease and multiple sclerosis.
Mobilized blood cells have been proposed as the source of CD34+ cells for gene therapy.33,34 Although a high transduction efficiency has been demonstrated in vitro,33 continued expression posttransplant remains low.34 There are still many technical hurdles associated with gene therapy,285 but mobilized blood cells do provide a larger number of progenitor cells than BM harvest could. Specific diseases or therapeutic indications that have been targeted include severe combined immune deficiency,286 P-glycoprotein expression,287 methotrexate resistance,288 chronic granulomatous disease,289 and Gaucher disease.290
SUMMARY
Mobilized blood cells produce faster hematopoietic and immune reconstitution than BM, making high-dose therapy safer and more cost effective. They are becoming the main cell source for autologous rescue and may provide an alternative in CML and myelodysplasia. Mobilized allogeneic blood cells have been used without severe GVHD while reproducing the rapid reconstitution seen in the autologous setting. The potential of using a high cell dose to transplant from mismatched or unrelated donors is exciting. Mobilized blood cells may yet replace BM in allogeneic transplants.
G-CSF alone (autologous and allogeneic) or a combination of chemotherapy and hematopoietic growth factors such as G-CSF and GM-CSF (autologous only) are the most commonly used mobilization protocols. They appear to be able to achieve adequate progenitor cell yields for single transplants in most patients except those who have been heavily pretreated. Indeed, a single apheresis may provide sufficient progenitor cells, especially in allogeneic donors. The design of mobilization and high-dose therapy protocols should incorporate mobilization early in the treatment process before BM exhaustion occurs. Adequate standardization of progenitor and stem cell measurement is necessary for reliable quantitation of hematopoietic reconstitutive capacity. Current data suggest the presence of minimum and optimum thresholds.
Blood cell transplants may have additional impact on tumor control. The large number of progenitor cells allows for multiple high-dose therapy and rescues. Tumor-directed purging and CD34+ cell (or its subset) selection are similarly facilitated. Lower tumor contamination of blood cells compared with BM has been suggested. The large number of accessory cells also allows for immunomodulatory approaches to enhance graft-versus-tumor response. None of these have been proven in phase III studies but are important research areas.
Major future advances may occur through better understanding of the mechanism of mobilization. This may provide a means of direct and therefore more predictable and higher-yield mobilization and also differential mobilization of normal and tumor cells. Technologic advances in ex vivo processing may also lead to more cost-effective high-dose therapy such as ambulatory transplants using expanded cells, tumor-free grafts, and genetically modified grafts. It is likely that mobilized blood will be used to treat nonmalignant diseases and as a vehicle for gene therapy. Lymphoid reconstitution may also provide an alternative means of treating autoimmune diseases.
ACKNOWLEDGMENT
The authors thank Ann Haylock for her tireless stenographic assistance, and Che To and Val and Garry Bickley for their review of manuscript.
Address reprint requests to L.B. To, MD, Division of Haematology, Hanson Centre, IMVS, Frome Rd, Adelaide SA 5000, Australia.