Abstract
The Shc gene encodes three proteins that have been implicated as mediators of signal transduction from growth factor receptors and nonreceptor tyrosine kinases to Ras. Overexpression of Shc in established murine fibroblasts results in oncogenic transformation, indicating that Shc has oncogenic potential. Shc proteins contain a carboxy terminal SH2 domain and a novel non-SH2 phosphotyrosine-binding (PTB) domain that specifically recognizes a phosphorylated NPXpY motif in target proteins such as the epidermal growth factor receptor. We show here that Shc is constitutively tyrosine-phosphorylated in all primary acute myeloid leukemias analyzed and that, in some of these leukemias, Shc is associated through its PTB domain with a tyrosinephosphorylated protein of 140 kD (p140) in vivo. In factor-dependent cells, this 140-kD protein can be tyrosine-phosphorylated in vitro in response to cytokines involved in myeloid proliferation and differentiation, ie, granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1. A similar or identical protein of 140 kD is constitutively bound to the C-terminal SH3 domain of Grb2 in the same acute myeloid leukemias. In addition to p140, other tyrosine-phosphorylated proteins of 61 and 200 kD are constitutively associated with Shc in some of the leukemias analyzed. Our results implicate Shc, Grb2, p140, and additional tyrosine-phosphorylated proteins of 61 and 200 kD in signalling of acute myeloid leukemia cells.
ACUTE MYELOID LEUKEMIA (AML) is characterized by the clonal expansion of hematopoietic progenitor cells that are blocked in terminal differentiation.1 The full leukemic phenotype of AML appears to be the result of several transforming events that cooperate in the process of leukemogenesis.2-4 In the last few years, genes that may be involved in the pathogenesis of AML have been identified by different approaches. The molecular cloning of translocation breakpoints associated with AML5,6 has identified the genes involved in the breakpoint junctions as transcriptional regulatory proteins such as AML1, ETO, EVI1, PML, and RARα.6-10 From these and additional data it has been concluded that transcription factors play an important role in the development of acute leukemias including AML, probably by blocking normal hematopoietic cell differentiation.4 11
Another major step in identifying genes that may contribute to AML came from the extensive work with hematopoietic growth factors (HGFs) and HGF receptors, which regulate the complex network of hematopoiesis.12,13 Several HGFs can induce or enhance the proliferation of AML cells in vitro, including granulocyte-macrophage colony-stimulating factor (GM-CSF ).14 An autocrine growth stimulation of AML cells by GM-CSF has been shown in some cases of AML.15-17 We have previously shown that coexpression of the α and β subunits of the human GM-CSF receptor in established murine fibroblasts can cause ligand-dependent transformation.18 However, mice constitutively expressing the GM-CSF or interleukin-3 (IL-3) gene develop myeloproliferative syndromes but not acute leukemia.19-21 These data suggest that the deregulated stimulation of hematopoietic cells with an HGF, eg, GM-CSF or IL-3, via autocrine or paracrine mechanisms can contribute to the leukemic transformation but that their unregulated expression alone is not sufficient to cause leukemia.
Mutated HGF receptors can have transforming activities that contribute to the development of leukemia in mice, as has been shown for the colony-stimulating factor-1 (CSF-1) receptor22 and erythropoietin (EPO) receptor.23 Mutations in the CSF-1 receptor gene have been described in about 16% of primary AML24 and constitutive tyrosine phosphorylation of c-Kit, which is the receptor for stem cell factor (SCF ), has been detected in 7 of 12 cases of AML.25 These data suggest that constitutively activated HGF receptors may be involved in the transformation of AML cells.
A deregulated growth stimulation of AML cells may also result from the constitutive activation of signal transduction pathways downstream of the HGF receptors. Mutations in ras genes,26,27 most often in N-ras, have been detected in about 25% of cases of AML.28-30 Ras is also activated in hematopoietic cells transformed by the p210Bcr/Abl fusion protein that is found in all cases of chronic myeloid leukemia.31 The prevention of apoptosis in hematopoietic cells by cytokine receptor stimulation may be mediated by Ras-dependent upregulation of bcl 2 gene expression.32,33 These and additional data implicate Ras proteins directly in leukemogenesis.34
Ras proteins become transiently activated after stimulation of receptor tyrosine kinases, cytokine receptors without an intrinsic tyrosine kinase domain, or by activated cytoplasmic tyrosine kinases.35-37 An increase in the active GTP-bound form of Ras can be induced either by activating the Ras nucleotide exchange factor SOS or by inhibiting the Ras GTPase activating protein.38 Activation of Ras by SOS involves the relocalization of SOS from the cytoplasm to the plasma membrane, which is mediated by the adaptor protein Grb2.39-41 Grb2 can bind constitutively to SOS, but the association of Grb2 with SOS can also be regulated, as has been shown after stimulation of the T-cell antigen receptor.42 Grb2 can bind directly to the activated epidermal growth factor (EGF ) receptor or bind indirectly via its association with tyrosine-phosphorylated Shc.43 44 Tyrosine phosphorylation of Shc proteins provides therefore an alternative mechanism to couple activated receptors and cytoplasmic tyrosine kinases to the Ras signalling pathway.
Shc adapter molecules can bind to activated receptor tyrosine kinases by interaction of their SH2 domain with distinct tyrosine-phosphorylated motifs in the receptor.44-46 Shc also contains a novel non-SH2 phosphotyrosine binding domain (PTB) located at its N terminus.47-50 Whereas binding of SH2 domains to specific motifs is controlled by residues carboxy terminal to the phosphotyrosine,51 the PTB domain selects its tyrosine-phosphorylated binding sites on the basis of residues amino terminal to the phosphotyrosine, ie, Asn-Pro-X-Tyr(P).46,50 The presence of a PTB domain in several otherwise unrelated regulatory proteins suggests a general role for this domain in signal transduction.52
Overexpression of Shc proteins transforms immortalized fibroblasts and increases the proliferative response of a myeloid leukemia cell line, indicating that Shc proteins have mitogenic and transforming potential.44,53 Constitutive phosphorylation of Shc proteins has been found in cells transformed by oncogenic tyrosine kinases54 and in chronic myeloid leukemia cells expressing the p210Bcr/Abl oncoprotein.55 Stimulation of cells with IL-3, GM-CSF, CSF-1, EPO, and SCF, which can sustain the proliferation of AML cells in vitro, leads to a transient tyrosine phosphorylation of Shc proteins.55-57 In this report, we present evidence that, in freshly isolated AML cells, Shc proteins are constitutively tyrosine-phosphorylated and associated with known and new signalling proteins, including a recently described 140-kD phosphorylated protein. We have further characterized the interactions between Shc and the Shc-associated proteins implicating the PTB domain in signalling of AML cells. Our results suggest a mechanism whereby the Ras pathway may be constitutively activated in AML.
MATERIALS AND METHODS
Cells.The GM-CSF–dependent human erythroleukemia cell line TF-158 was obtained from A. Miyajima (DNAX Research Institute, Palo Alto, CA). These cells were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% (vol/vol) fetal bovine serum and 3 ng/mL recombinant human GM-CSF (hGM-CSF ). Control and v-fes–transformed NIH 3T3 cells have been described.59 BAC1.2F5 is a macrophage cell line that is dependent on CSF-1 for survival and proliferation.60 BAC1.2F5 cells were maintained in α minimal essential medium (α-MEM) supplemented with 10% (vol/vol) fetal bovine serum (GIBCO) and 36 ng/mL of human recombinant CSF-1 (Chiron Corp, Emeryville, CA). Leukemia cells were obtained from the peripheral blood of patients with AML and chronic lymphocytic leukemia. The diagnosis in each case was made by morphology, cytochemistry, and surface antigen analysis. Normal peripheral blood cells were obtained from a healthy donor. Mononuclear peripheral blood cells from leukemia patients and from a healthy donor were fractionated by gradient sedimentation using Histopaque-1077 according to the manufacturer (Sigma, St Louis, MO). After gradient sedimentation, the percentage of blasts was more than 90% in each sample. Fractionated leukemia cells were then lysed without further handling.
Antibodies.Rat monoclonal antibody (MoAb) CRS1 directed against the β subunit of the hGM-CSF receptor has been previously described61 and was obtained from A. Miyajima. An MoAb directed against Grb2 was purchased from Transduction Laboratories (Lexington, KY). MoAb EL6 against Grb2 was obtained from E. Lowenstein and J. Schlessinger.62 Rabbit antisera directed against Shc and Jak2 and MoAb directed against phosphotyrosine (4G10) and phospholipase C-γ (PLC-γ) were obtained from UBI (Lake Placid, NY).
Stimulation with hGM-CSF.TF-1 cells were starved for 18 hours in RPMI 1640 medium containing 0.1% (wt/vol) bovine serum albumin (BSA; fraction V; Sigma) at a cell density of 5 × 105 cells/mL. Cells were then resuspended in fresh medium containing 0.1% (wt/vol) BSA at a cell density of 1 × 107 cells/mL and were then incubated for 2 minutes at 37°C in the absence or presence of 25 ng/mL hGM-CSF, unless otherwise indicated. After incubation, cells were diluted into cold phosphate-buffered saline containing 1 mmol/L sodium orthovanadate and cell lysates were prepared as described below. The stimulation of BAC1.2F5 cells with 35 ng/mL CSF-1 was performed as previously described.63
Preparation of cell lysates and protein analysis.Untreated or hGM-CSF–treated cells were lysed in NP-40 lysis buffer (50 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 1% [vol/vol] NP-40, 2 mmol/L EDTA, 10% [vol/vol] glycerol, 50 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 1 mmol/L sodium orthovanadate, and 2% [vol/vol] Trasylol [FBA Pharmaceuticals, New York, NY]). Insoluble material was removed by centrifugation at 4°C for 10 minutes at 14,000g. Protein concentration was determined using the BioRad protein assay according to the manufacturer (BioRad, Melville, NY), and cell lysates were stored in liquid nitrogen until used.
Cell lysates were immunoprecipitated with the indicated antibodies and the immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE and Western blot analysis were performed as described previously.63 The concentration of the MoAbs or rabbit sera used for Western blot analyses were 0.5 μg/mL of antiphosphotyrosine MoAb 4G10 and anti–PLC-γ1 MoAb, 1 μg/mL of anti-Shc MoAb, and 1:500 dilution for anti-GRB2 MoAb. Filters were developed using the enhanced chemiluminescence (ECL) system according to the manufacturer (Amersham, Arlington Heights, IL). Reprobing of nitrocellulose membrane was performed as described previously.64
Purification of bacterial fusion proteins.The Shc-SH2 domain was expressed as a fusion protein with glutathione S-transferase (GST) after subcloning a DNA fragment corresponding to nucleotides 1214-1495 of the Shc cDNA into pGEX-2T. The N-terminal domain of Shc (amino acids 1 to 273) containing the PTB domain was expressed as a fusion protein with maltose-binding protein (MBP). pGEX-3X control and pGEX-fusion proteins were induced with 0.1 mmol/L Isopropyl-β-D-thiogalacto-pyranoside (IPTG), the induced bacteria were lysed by sonication in a buffer containing 1% (vol/vol) Triton X-100, 50 mmol/L HEPES (pH 7.4), and the induced proteins were adsorbed with glutathione-agarose (Sigma), as described previously.63 Expression of MBP and MBP fusion proteins was induced with 0.3 mmol/L IPTG in the presence of 0.2% (vol/vol) glucose for 2 hours at 37°C. Bacteria were lysed as described above and the MBP fusion proteins were adsorbed with amylose resin (New England Biolabs, Beverly, MA).
RESULTS
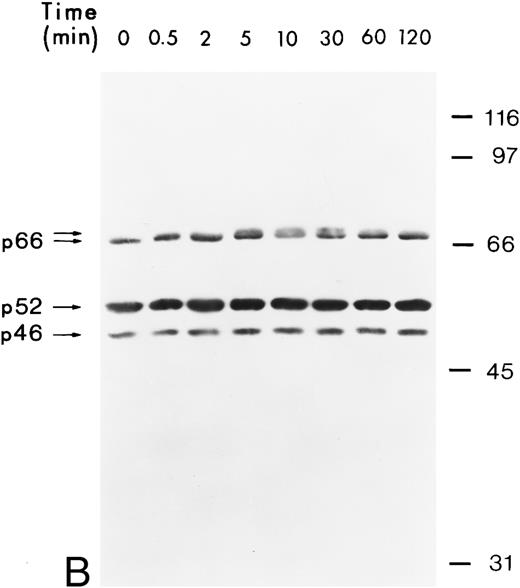
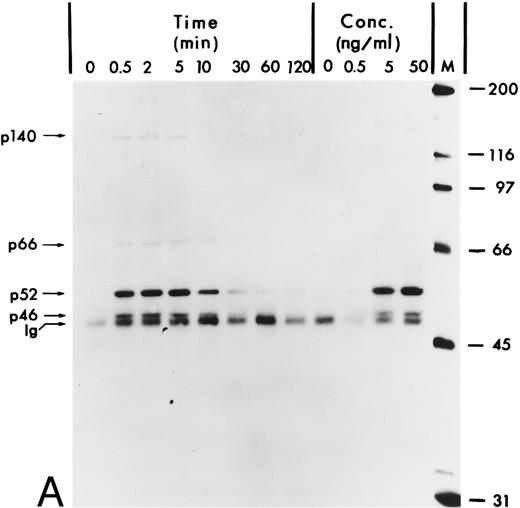
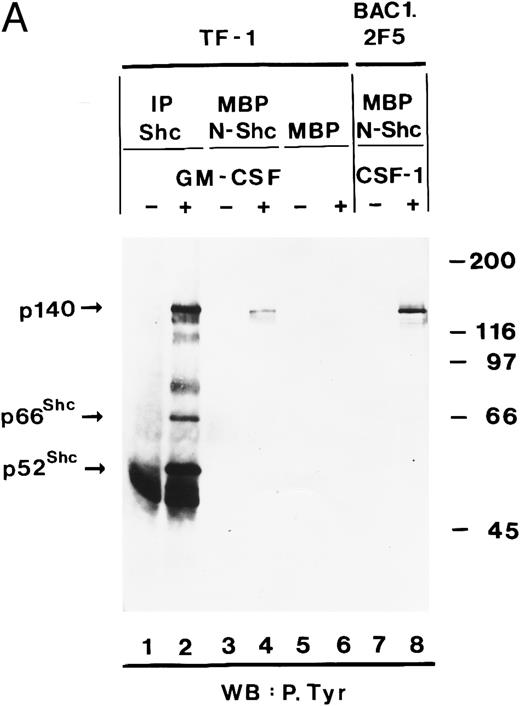
hGM-CSF induces tyrosine phosphorylation of Shc proteins and their association with a 140-kD phosphotyrosyl protein.The Shc proteins are known to be transiently phosphorylated on tyrosine after ligand stimulation of cytokine receptors present in myeloid cells. Therefore, we wanted to compare the state of phosphorylation of Shc proteins in cells in which its phosphorylation is ligand-dependent with that in AML, in which autocrine or paracrine mechanisms may result in deregulated phosphorylation of Shc. Our analysis of Shc proteins in the GM-CSF–dependent human erythroleukemia cell line TF-1 showed that, after hGM-CSF treatment, all three Shc proteins p46Shc, p52Shc, and p66Shc become rapidly and transiently phosphorylated on tyrosine (Fig 1A). The major tyrosine-phosphorylated Shc protein was p52Shc, which was also the most abundant form in TF-1 cells (Fig 1B). Maximal tyrosine phosphorylation of all three Shc proteins occurred within 2 minutes, remained at the maximum for up to 5 minutes, and declined to very low (p52Shc) or undetectable (p46Shc and p66Shc) levels 2 hours after hGM-CSF stimulation. Tyrosine phosphorylation of Shc proteins was dependent on the concentration of hGM-CSF, with maximal stimulation at 5 ng/mL (Fig 1A). Anti-Shc immunoprecipitates from hGM-CSF–treated TF-1 cells contained a 140-kD phosphotyrosyl protein (p140; Fig 1A). p140 was not directly recognized by anti-Shc antibody in immunoblots (Fig 1B), indicating that p140 was an associated protein different from Shc. The time courses of coprecipitation of phosphotyrosyl p140 and tyrosine phosphorylation of Shc proteins were identical, suggesting that the direct or indirect association between Shc and p140 may involve phosphotyrosine-dependent interactions (Fig 1A).
Tyrosine phosphorylation of Shc proteins in hGM-CSF–stimulated TF-1 cells and identification of an associated 140-kD phosphotyrosyl protein. (A) Starved TF-1 cells were stimulated for the indicated times with 25 ng/mL hGM-CSF or with the indicated concentrations of hGM-CSF for 5 minutes at 37°C. Cells were lysed and aliquots containing 1 mg protein were immunoprecipitated with anti-Shc antibodies. Immunoprecipitates were subjected to Western blot analysis with antiphosphotyrosine antibodies. The positions of the Shc proteins (p46, p52, and p66) and p140 are indicated. The apparent molecular weights of the Shc proteins p46Shc, p52Shc, and p66Shc in our gel system were consistently 51, 56, and 68 kD. To avoid confusion, we will continue to name the Shc proteins as p46, p52, and p66 according to Pelicci et al.44 (B) Starved TF-1 cells were stimulated for the indicated times with hGM-CSF. Cells were lysed and total cell lysates containing 50 μg of protein were subjected to Western blot analysis using anti-Shc antibodies. The positions of Shc proteins (p46, p52, and p66) are indicated. The arrow above p66 indicates a slower migrating form of p66 detected during hGM-CSF stimulation.
Tyrosine phosphorylation of Shc proteins in hGM-CSF–stimulated TF-1 cells and identification of an associated 140-kD phosphotyrosyl protein. (A) Starved TF-1 cells were stimulated for the indicated times with 25 ng/mL hGM-CSF or with the indicated concentrations of hGM-CSF for 5 minutes at 37°C. Cells were lysed and aliquots containing 1 mg protein were immunoprecipitated with anti-Shc antibodies. Immunoprecipitates were subjected to Western blot analysis with antiphosphotyrosine antibodies. The positions of the Shc proteins (p46, p52, and p66) and p140 are indicated. The apparent molecular weights of the Shc proteins p46Shc, p52Shc, and p66Shc in our gel system were consistently 51, 56, and 68 kD. To avoid confusion, we will continue to name the Shc proteins as p46, p52, and p66 according to Pelicci et al.44 (B) Starved TF-1 cells were stimulated for the indicated times with hGM-CSF. Cells were lysed and total cell lysates containing 50 μg of protein were subjected to Western blot analysis using anti-Shc antibodies. The positions of Shc proteins (p46, p52, and p66) are indicated. The arrow above p66 indicates a slower migrating form of p66 detected during hGM-CSF stimulation.
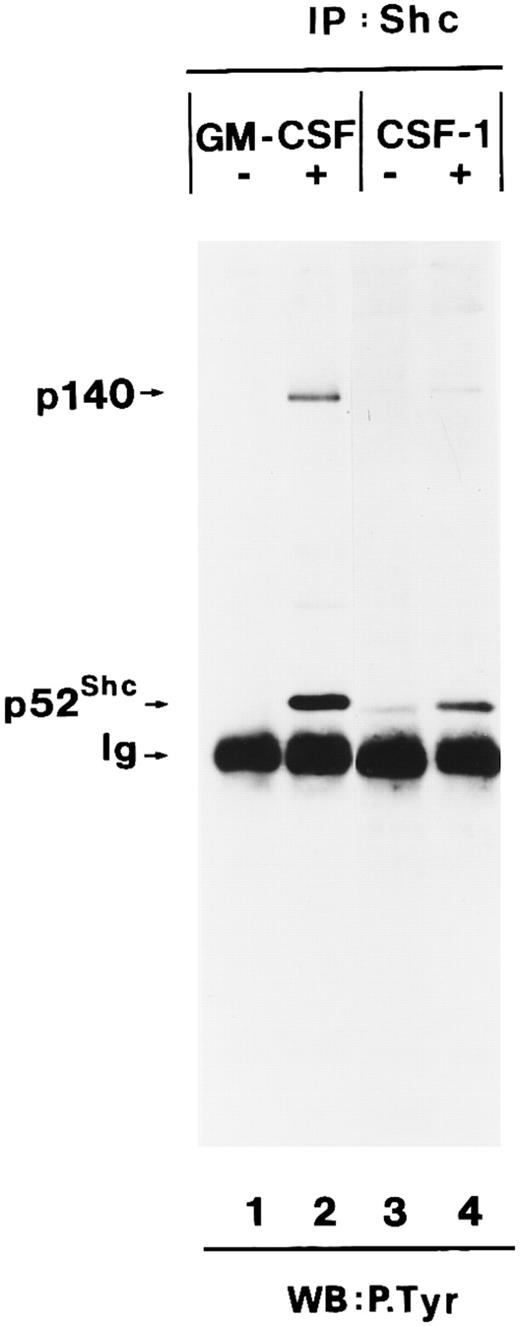
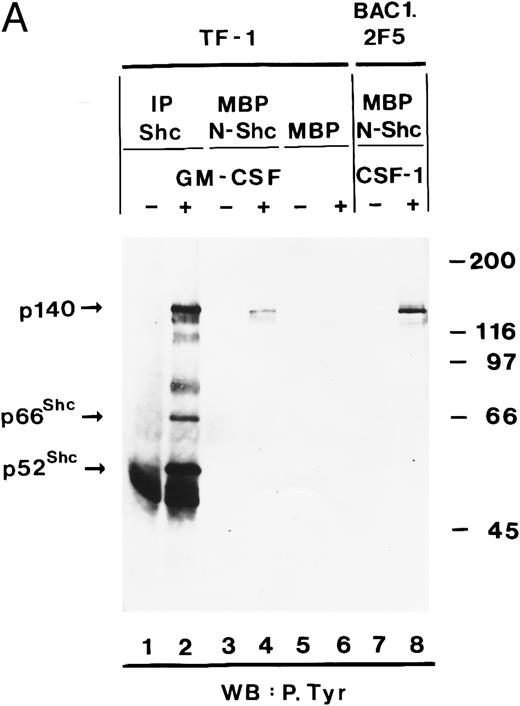
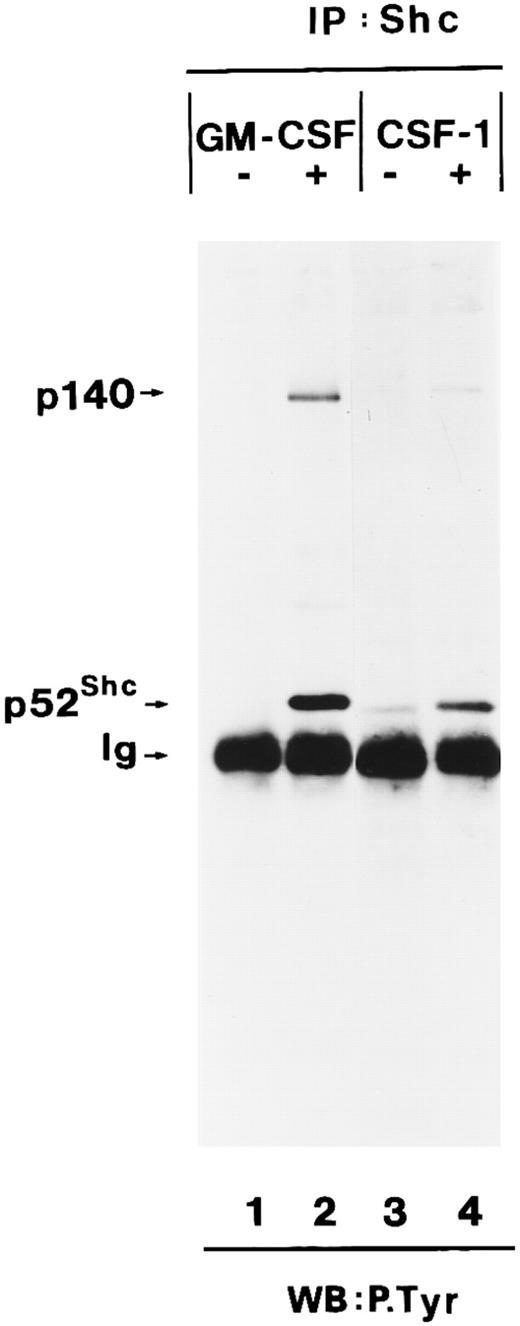
We then determined whether tyrosine-phosphorylated p140 could also be detected after stimulation of growth factor receptors with intrinsic tyrosine kinase domains such as the CSF receptor. CSF-1 treatment of BAC1.2F5 cells resulted in tyrosine phosphorylation of a similar or identical 140-kD protein and its association with tyrosine-phosphorylated Shc (Fig 2, lanes 3 and 4).
Association of tyrosine-phosphorylated p140 with Shc in response to different growth factors. Anti-Shc immunoprecipitates (IP:Shc) of unstimulated (lane 1) or hGM-CSF–stimulated (lane 2) TF-1 cells and from unstimulated (lane 3) or CSF-1–stimulated (lanes 4) BAC1.2F5 cells were analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 4). The positions of p140, p52Shc, and Ig heavy chain (Ig) are indicated on the left.
Association of tyrosine-phosphorylated p140 with Shc in response to different growth factors. Anti-Shc immunoprecipitates (IP:Shc) of unstimulated (lane 1) or hGM-CSF–stimulated (lane 2) TF-1 cells and from unstimulated (lane 3) or CSF-1–stimulated (lanes 4) BAC1.2F5 cells were analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 4). The positions of p140, p52Shc, and Ig heavy chain (Ig) are indicated on the left.
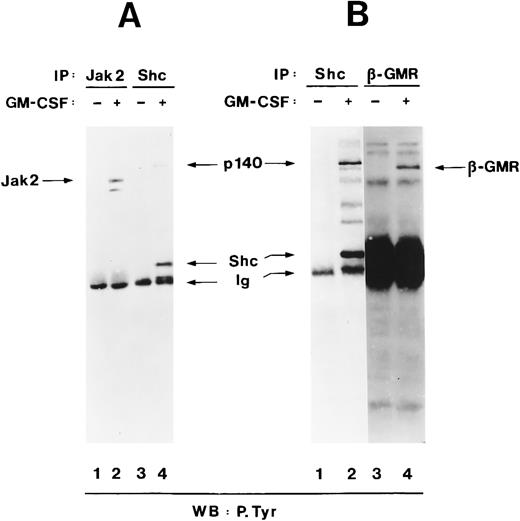
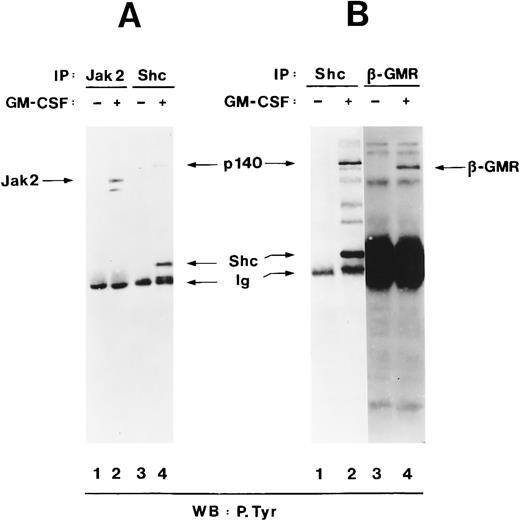
To examine whether p140 was a known signalling protein, we compared its electrophoretic mobility with that of other signalling proteins in the same molecular weight range, namely Jak2 and the β subunit of the hGM-CSF receptor (hGMR), which also become tyrosine-phosphorylated in response to hGM-CSF treatment.65-67 In TF-1 cells, Jak2 was tyrosine-phosphorylated after hGM-CSF stimulation (Fig 3A, lanes 1 and 2), but Jak2 migrated with an apparent molecular weight of 120 kD, indicating that p140 was not Jak2 (Fig 3A, lane 4). Stripping and reprobing of the filter shown in Fig 3A with anti-Jak2 antibodies showed that similar amounts of Jak2 were precipitated from unstimulated and hGM-CSF–stimulated cells (data not shown). Similarly, tyrosine-phosphorylated β, which had an apparent molecular weight of 130 kD, did not comigrate with p140 either (Fig 3B, lanes 2 and 4). Interestingly, a 130-kD protein that comigrated with the β subunit of hGMR was also detectable in the anti-Shc immunoprecipitate (Fig 3B, lane 2), suggesting that Shc might associate with the β subunit directly or indirectly.
p140 is a new phosphotyrosyl protein. (A) Cell lysates from unstimulated (lanes 1 and 3) or hGM-CSF–stimulated (lanes 2 and 4) TF-1 cells were immunoprecipitated with anti-Jak2 (lane 1 and 2) or anti-Shc (lanes 3 and 4) antibodies and analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 4). The positions of Jak2, Shc, p140, and Ig heavy chain (Ig) are indicated. (B) Cell lysates from unstimulated (lanes 1 and 3) or hGM-CSF–stimulated (lane 2 and 4) TF-1 cells were immunoprecipitated with antibodies directed against Shc (lanes 1 and 2) or the β subunit of the human GM-CSF receptor (β-GMR; lanes 3 and 4) and analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 4).
p140 is a new phosphotyrosyl protein. (A) Cell lysates from unstimulated (lanes 1 and 3) or hGM-CSF–stimulated (lanes 2 and 4) TF-1 cells were immunoprecipitated with anti-Jak2 (lane 1 and 2) or anti-Shc (lanes 3 and 4) antibodies and analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 4). The positions of Jak2, Shc, p140, and Ig heavy chain (Ig) are indicated. (B) Cell lysates from unstimulated (lanes 1 and 3) or hGM-CSF–stimulated (lane 2 and 4) TF-1 cells were immunoprecipitated with antibodies directed against Shc (lanes 1 and 2) or the β subunit of the human GM-CSF receptor (β-GMR; lanes 3 and 4) and analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 4).
We also determined that PLC-γ, which has an apparent molecular weight of 145 kD, was not tyrosine-phosphorylated in hGM-CSF–stimulated cells, did not associate with Shc proteins after hGM-CSF stimulation, and did not comigrate with p140 (data not shown). These results exclude an identity of PLC-γ with p140 and suggest that PLC-γ may not be activated by hGM-CSF in these cells.
We conclude that p140 is a new phosphotyrosyl protein involved in hGM-CSF and CSF-1 signalling. p140 may act in concert with Shc and Grb2, as described below in more detail.
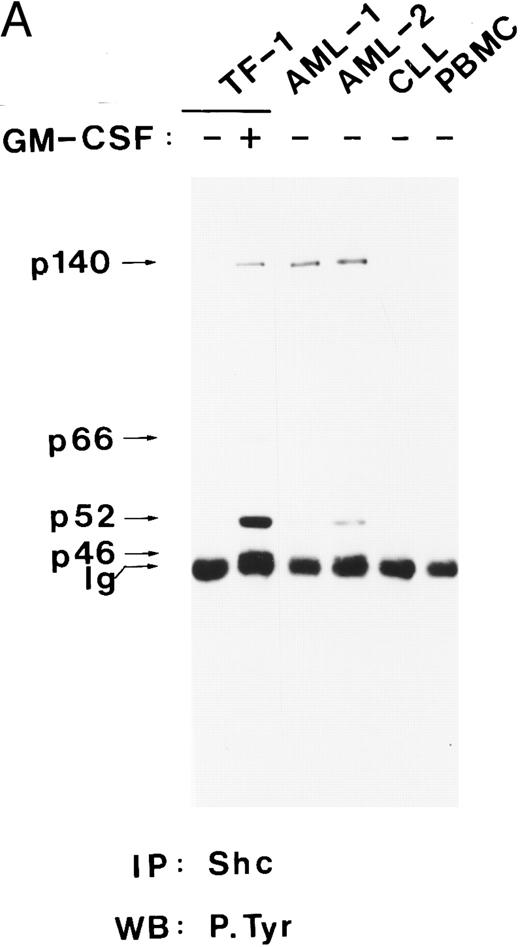
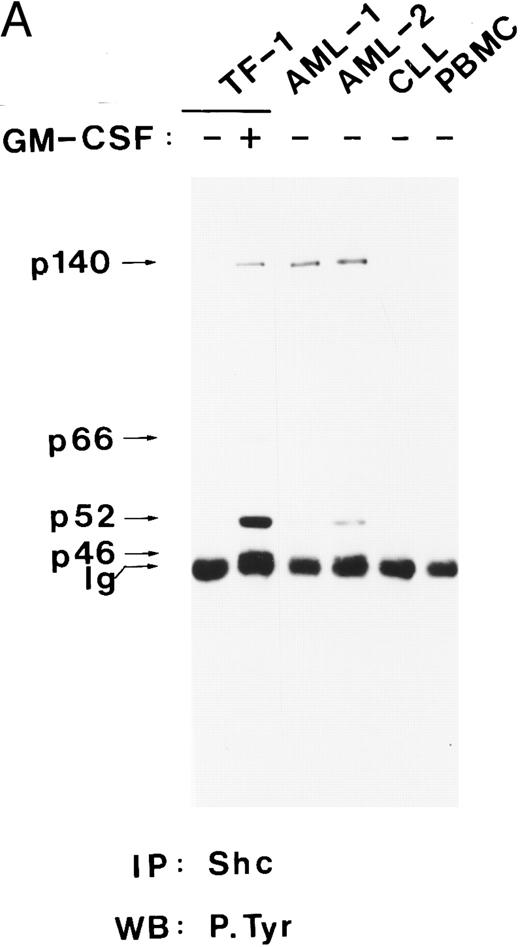
Shc and p140 are constitutively tyrosine-phosporylated in AML cells.The Shc proteins are constitutively tyrosine phosphorylated in cells transformed by v-src and v-fps,54 and their overexpression can cause transformation in NIH 3T3 cells,44 suggesting that Shc activation may be a step in neoplastic transformation. Therefore, we wanted to examine the state of phosphorylation of Shc proteins in myeloid leukemia cells. Our analysis showed that p52Shc was constitutively tyrosine phosphorylated at different levels in freshly isolated peripheral blood cells from eight of eight AML patients but not in peripheral blood cells from a chronic lymphocytic leukemia (CLL) patient or in peripheral blood mononuclear cells (PBMC) from a healthy donor (Fig 4A and B). Whether p46Shc was also constitutively phosphorylated in the leukemia samples could not be determined because p46Shc comigrated with the Ig band. For AML-4 and AML-8, for which bone marrow cells were available, constitutively tyrosine-phosphorylated Shc proteins were detected in both the peripheral blood and the bone marrow cells (data not shown). Reprobing of the stripped antiphosphotyrosine blots with anti-Shc antibody showed that p46Shc and p52Shc were expressed in all samples analyzed, whereas the 66-kD isoform of Shc was expressed in AML-3 only (data not shown).
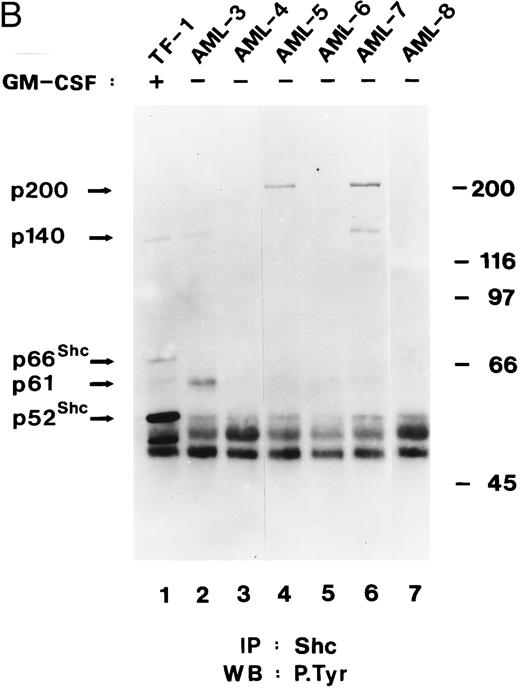
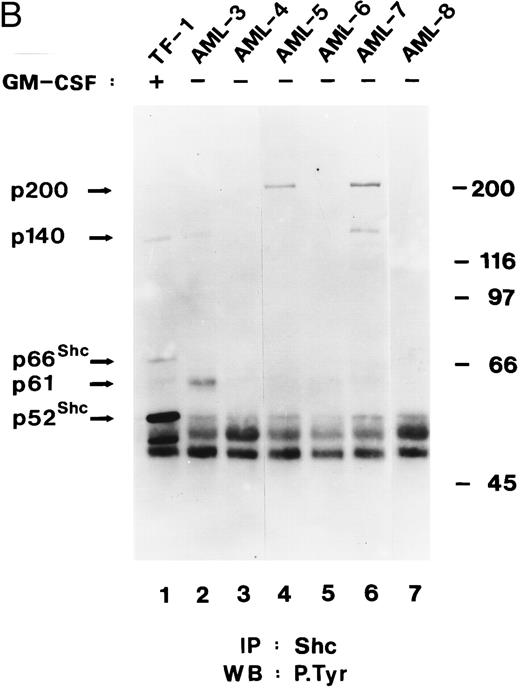
Shc and p140 are constitutively phosphorylated on tyrosine in AML cells. (A) Cell lysates from unstimulated (−) or hGM-CSF–stimulated (+) TF-1 cells and lysates of primary leukemia cells obtained from two patients with AML (AML-1 and AML-2), CLL, and a lysate of PBMCs obtained from a healthy donor were immunoprecipitated with anti-Shc antibodies and subjected to antiphosphotyrosine immunoblotting (P.Tyr). (B) Anti-Shc immunoprecipitates of lysates from hGM-CSF–stimulated TF-1 cells (lane 1) and primary AML (AML-3 through AML-8; lanes 2 through 7) were analyzed by antiphosphotyrosine immunoblotting. The positions of p52Shc, p66Shc, p61, p140, and p200 are indicated on the left. Molecular weight marker bands are indicated on the right.
Shc and p140 are constitutively phosphorylated on tyrosine in AML cells. (A) Cell lysates from unstimulated (−) or hGM-CSF–stimulated (+) TF-1 cells and lysates of primary leukemia cells obtained from two patients with AML (AML-1 and AML-2), CLL, and a lysate of PBMCs obtained from a healthy donor were immunoprecipitated with anti-Shc antibodies and subjected to antiphosphotyrosine immunoblotting (P.Tyr). (B) Anti-Shc immunoprecipitates of lysates from hGM-CSF–stimulated TF-1 cells (lane 1) and primary AML (AML-3 through AML-8; lanes 2 through 7) were analyzed by antiphosphotyrosine immunoblotting. The positions of p52Shc, p66Shc, p61, p140, and p200 are indicated on the left. Molecular weight marker bands are indicated on the right.
In 4 of 8 AML samples (AML-1, AML-2, AML-3, and AML-7), immunoprecipitation of Shc resulted in coprecipitation of a tyrosine-phosphorylated protein of 140 kD (Fig 4A and B). This 140-kD protein had the same electrophoretic mobility as the 140-kD protein coprecipitating with Shc in hGM-CSF–stimulated TF-1 cells (Fig 4A). Two additional tyrosine-phosphorylated proteins of 61 kD and 200 kD coprecipitating with Shc in AML cell lysates were discovered during this analysis (Fig 4B, lanes 2, 4, and 6). The tyrosine-phosphorylated 61-kD protein (p61) was detected in one leukemia sample (AML-3; Fig 4B, lane 2) and the 200-kD protein (p200) was detected in two other AML samples (AML-5 and AML-7; Fig 4B, lanes 4 and 6). In one of these leukemias (AML-7), p200 and p140 were found to coprecipitate with Shc (Fig 4B, lane 6). However, in AML-5, p200 was the only coprecipitating tyrosine-phosphorylated protein, suggesting that Shc and p200 can form a complex without tyrosine-phosphorylated p140 (Fig 4B, lane 4).
These results indicate that Shc is constitutively tyrosine-phosphorylated in all AML samples analyzed and that Shc is constitutively associated with p140 and additional tyrosine-phosphorylated proteins of 61 kD and 200 kD in some AML.
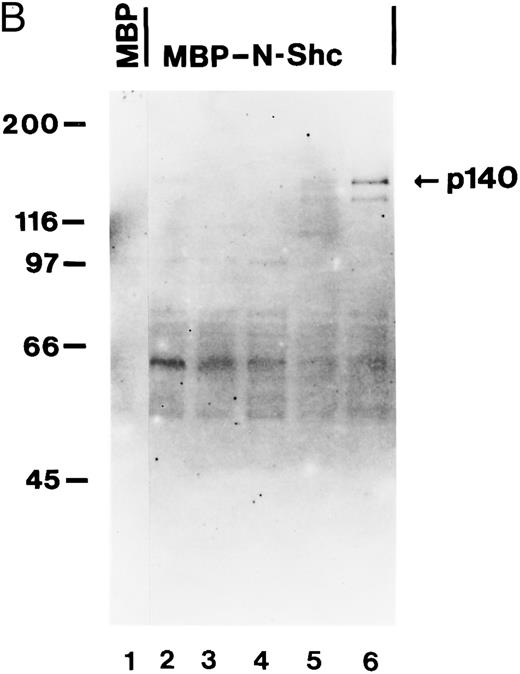
p140 is constitutively associated with the phosphotyrosine binding domain of Shc in AML cells.To further analyze the association between Shc and p140 in more detail, we used bacterial fusion proteins. Two domains of Shc have been shown to interact with phosphotyrosine, ie, the SH2 domain, and the N-terminus containing the PTB domain. The SH2 domain was expressed as a GST fusion protein and the PTB domain as a MBP fusion protein. First we used TF-1 cells as a model system, in which hGM-CSF induced association of Shc with p140 (Fig 1A). Cell lysates of unstimulated and hGM-CSF–stimulated TF-1 cells were incubated with the MBP fusion protein containing the PTB domain of Shc (MBP-N-Shc). Proteins bound to the fusion protein were purified by affinity chromatography on amylose resin and further analyzed by SDS-PAGE and antiphosphotyrosine immunoblotting. As shown in Fig 5A, a tyrosine-phosphorylated protein of 140 kD was recognized by the PTB domain of Shc in a hGM-CSF–dependent manner (Fig 5A, lanes 3 and 4), whereas MBP vector protein did not bind any phosphotyrosyl proteins (Fig 5A, lanes 5 and 6). This 140-kD protein comigrated with the 140-kD protein detected in anti-Shc immunoprecipitates (Fig 5A, lane 2). A similar or identical 140-kD protein was also found to bind to the PTB domain of Shc in a macrophage cell line (BAC1.2F5) after stimulation with CSF-1 (Fig 5A, lanes 7 and 8). When we performed similar experiments using a GST fusion protein containing the SH2 domain of Shc, we were not able to detect any binding of the Shc SH2 domain to p140 (data not shown).
The association between Shc and p140 is mediated through the PTB domain of Shc and this complex is constitutively activated in some AML. (A) hGM-CSF and CSF-1 induce the association of tyrosine-phosphorylated p140 with the PTB domain of Shc. Cell lysates from unstimulated (lanes 1, 3, and 5) or hGM-CSF–stimulated (lanes 2, 4, and 6) TF-1 cells or from unstimulated (lane 7) or CSF-1-stimulated (lane 8) BAC1.2F5 cells were immunoprecipitated with anti-Shc antibodies (lanes 1 and 2) or adsorbed with either MBP vector (lanes 5 and 6) or MBP fusion proteins containing the N-terminal PTB domain of Shc (MBP-N-Shc; lanes 3, 4, 7, and 8). Associated tyrosine-phosphorylated proteins were analyzed by antiphosphotyrosine immunoblotting. (B) p140 is constitutively associated with the PTB domain of Shc in AML. Cell lysates of unstimulated primary AML were either incubated with MBP vector protein (AML-3; lane 1) or MBP-N-Shc fusion protein (AML 3 through AML-7; lanes 2 through 6), and the adsorbed proteins were analyzed by antiphosphotyrosine immunoblotting. The AML samples analyzed in this experiment correspond to the AML-3 through AML-7 samples analyzed in Fig 4B, lanes 2 through 6.
The association between Shc and p140 is mediated through the PTB domain of Shc and this complex is constitutively activated in some AML. (A) hGM-CSF and CSF-1 induce the association of tyrosine-phosphorylated p140 with the PTB domain of Shc. Cell lysates from unstimulated (lanes 1, 3, and 5) or hGM-CSF–stimulated (lanes 2, 4, and 6) TF-1 cells or from unstimulated (lane 7) or CSF-1-stimulated (lane 8) BAC1.2F5 cells were immunoprecipitated with anti-Shc antibodies (lanes 1 and 2) or adsorbed with either MBP vector (lanes 5 and 6) or MBP fusion proteins containing the N-terminal PTB domain of Shc (MBP-N-Shc; lanes 3, 4, 7, and 8). Associated tyrosine-phosphorylated proteins were analyzed by antiphosphotyrosine immunoblotting. (B) p140 is constitutively associated with the PTB domain of Shc in AML. Cell lysates of unstimulated primary AML were either incubated with MBP vector protein (AML-3; lane 1) or MBP-N-Shc fusion protein (AML 3 through AML-7; lanes 2 through 6), and the adsorbed proteins were analyzed by antiphosphotyrosine immunoblotting. The AML samples analyzed in this experiment correspond to the AML-3 through AML-7 samples analyzed in Fig 4B, lanes 2 through 6.
We then analyzed whether the constitutive association of Shc to p140 in primary AML was mediated through the PTB domain of Shc. After incubation of cell lysates from five AML patients (AML-3 through AML-7) with the MBP-N-Shc fusion protein immobilized on amylose resin, a 140-kD phosphotyrosine protein was detected in AML-7 (Fig 5B, lane 6) and was weakly detected in AML-3 and AML-6 (Fig 5B, lanes 2 and 5, respectively). In the case of AML-6, we were not able to detect p140 in anti-Shc immunoprecipitates (Fig 4B, lane 5), suggesting that the amount of p140 that can be purified with a bacterially expressed Shc fusion proteins is higher than by coimmunoprecipitation with anti-Shc antibodies. These data indicate that in these three AML samples Shc is constitutively associated with p140 and that this association is mediated through the PTB domain of Shc.
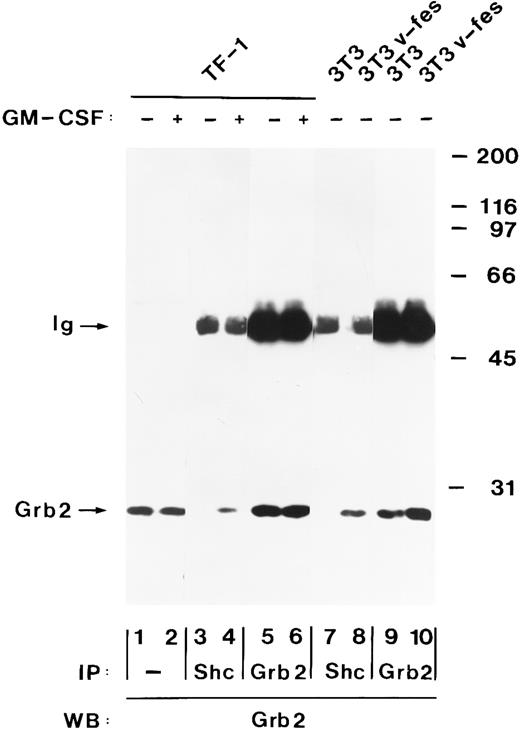
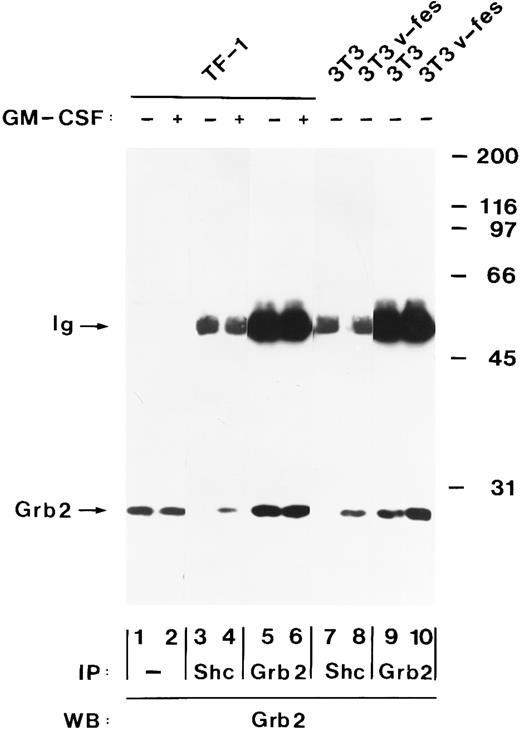
Tyrosine-phosphorylated Shc and p140 form a complex with Grb2 after hGM-CSF stimulation.Grb2 is an SH2/SH3 adapter protein that links receptor tyrosine kinases to the Ras pathway.40 Tyrosine-phosphorylated Shc proteins bind to the SH2 domain of Grb2 after stimulation of cells with growth factors and in cells transformed by oncogenic tyrosine kinases.37 44 To determine if Shc also associates with Grb2 after hGM-CSF stimulation, immunoprecipitates of Shc were analyzed for the presence of Grb2 by immunoblot analysis. Grb2 was present in anti-Shc immunoprecipitates from hGM-CSF–stimulated but not from unstimulated TF-1 cells (Fig 6, lanes 3 and 4). The amount of Grb2 present in the Shc immunoprecipitates was a fraction of the total Grb2 that was precipitated by Grb2 antibodies (Fig 6, lane 5 and 6), indicating that only part of Grb2 formed a complex with Shc after hGM-CSF stimulation. Stripping of the filter and reprobing with anti-Shc antiserum showed that equal amounts of Shc were precipitated by the antibody (data not shown). In v-fes–transformed NIH 3T3 cells, which were used as a positive control, Shc was also associated with Grb2, whereas no association was detected in control NIH 3T3 cells (Fig 6, lanes 7 through 10).
hGM-CSF induces the association of tyrosine-phosphorylated Shc with Grb2. Cell lysates from unstimulated (lanes 1, 3, and 5) or hGM-CSF–stimulated TF-1 cells (lanes 2, 4, and 6) and from control NIH 3T3 (lanes 7 and 9) or v-fes–transformed NIH 3T3 cells (lanes 8 and 10) were analyzed either directly (lanes 1 and 2) or after immunoprecipitation with anti-Shc (lanes 3, 4, 7, and 8) or anti-Grb2 (lanes 5, 6, 9, and 10) antibodies. Total cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting using anti-Grb2 antibodies (lanes 1 through 10). The positions of Grb2 and the Ig heavy chain (Ig) are indicated on the left. Molecular weight markers are indicated on the right.
hGM-CSF induces the association of tyrosine-phosphorylated Shc with Grb2. Cell lysates from unstimulated (lanes 1, 3, and 5) or hGM-CSF–stimulated TF-1 cells (lanes 2, 4, and 6) and from control NIH 3T3 (lanes 7 and 9) or v-fes–transformed NIH 3T3 cells (lanes 8 and 10) were analyzed either directly (lanes 1 and 2) or after immunoprecipitation with anti-Shc (lanes 3, 4, 7, and 8) or anti-Grb2 (lanes 5, 6, 9, and 10) antibodies. Total cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting using anti-Grb2 antibodies (lanes 1 through 10). The positions of Grb2 and the Ig heavy chain (Ig) are indicated on the left. Molecular weight markers are indicated on the right.
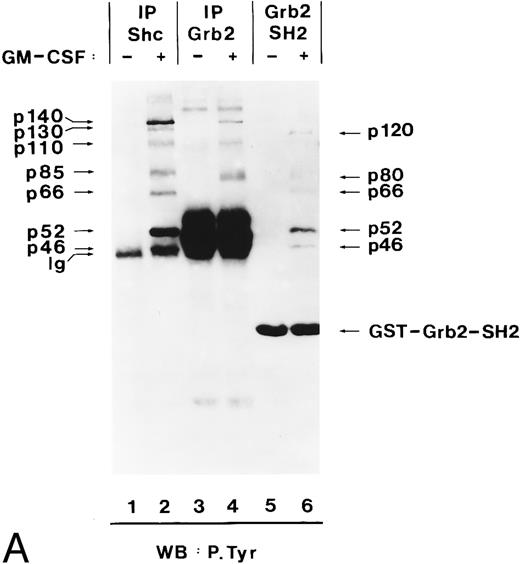
In EGF-stimulated cells, tyrosine-phosphorylated Shc binds to Grb2 through the Grb2 SH2 domain.37 Using the Grb2 SH2 domain expressed as a GST fusion protein, we analyzed whether in hGM-CSF–stimulated TF-1 cells binding of Shc to Grb2 was also mediated through the SH2 domain of Grb2. The Grb2 SH2 domain recognized tyrosine-phosphorylated proteins of 51, 56, and 68 kD (Fig 7A, lane 6), which had the same electrophoretic mobility as p46Shc, p52Shc, and p66Shc (Fig 7A, lane 2). Their identity as the three Shc proteins was confirmed by stripping and reprobing of the same filter with anti-Shc antibodies (data not shown). Two other unknown tyrosine-phosphorylated proteins of 80 and 120 kD were also found to bind to the SH2 domain of Grb2 in hGM-CSF–stimulated cells (Fig 7A, lane 6).
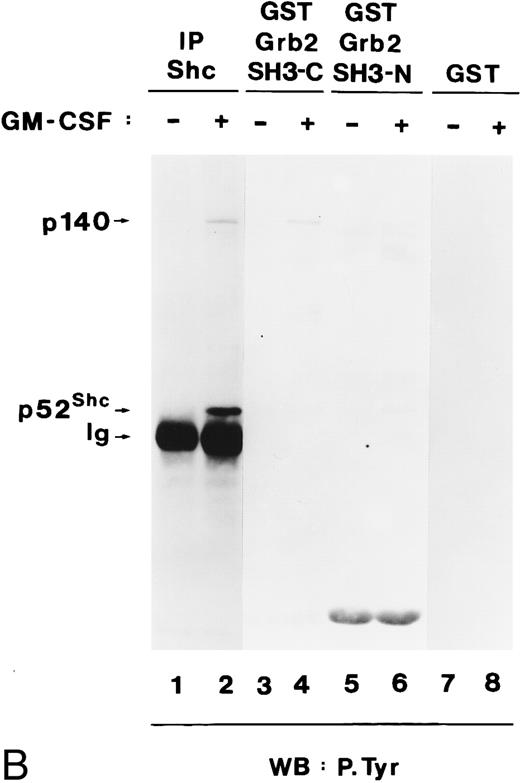
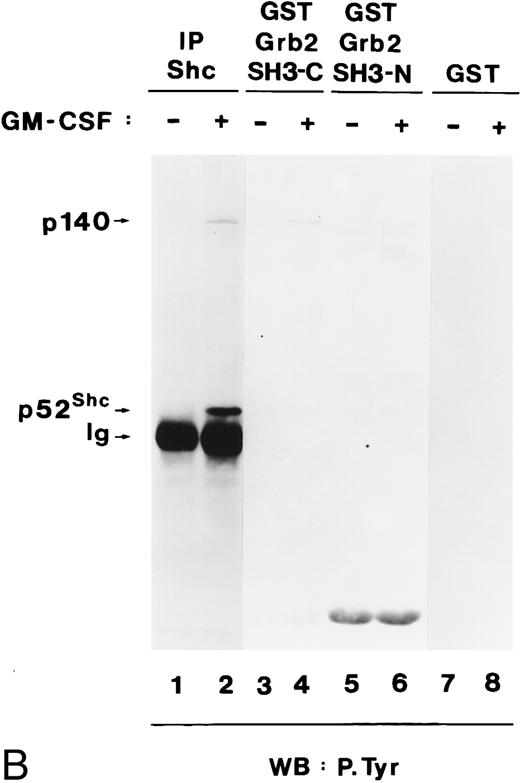
Grb2 associates through its SH2 domain with Shc and through its SH3 domain with p140, and both interactions are hGM-CSF–dependent. (A) Binding of Grb2 to tyrosine-phosphorylated Shc is mediated through the SH2 domain of Grb2. Cell lysates from unstimulated (lanes 1, 3, and 5) or hGM-CSF–stimulated (lanes 2, 4, and 6) TF-1 cells were immunoprecipitated (IP) with anti-Shc (lanes 1 and 2) or anti-Grb2 (lanes 3 and 4) antibodies or adsorbed with a GST fusion protein containing the SH2 domain of Grb2 (Grb2 SH2; lanes 5 and 6). The precipitates were analyzed by SDS-PAGE followed by antiphosphotyrosine immunoblotting (lanes 1 through 6). Arrows indicate the position of the detected phosphotyrosyl proteins and the GST-Grb2-SH2 fusion protein. (B) Binding of Grb2 to tyrosine-phosphorylated p140 is mediated through the SH3 domains of Grb2. Cell lysates of unstimulated (lanes 1, 3, 5, and 7) or hGM-CSF–stimulated (lanes 2, 4, 6, and 8) TF-1 cells were immunoprecipitated with anti-Shc antibodies (lanes 1 and 2) or adsorbed with either a GST fusion protein containing the C-terminal SH3 domain of Grb2 (GST Grb2 SH3-C; lanes 3 and 4), the N-terminal SH3 domain of Grb2 (GST Grb2 SH3-N; lanes 5 and 6), or with GST vector protein (GST) (lanes 7 and 8). The precipitates were analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 8).
Grb2 associates through its SH2 domain with Shc and through its SH3 domain with p140, and both interactions are hGM-CSF–dependent. (A) Binding of Grb2 to tyrosine-phosphorylated Shc is mediated through the SH2 domain of Grb2. Cell lysates from unstimulated (lanes 1, 3, and 5) or hGM-CSF–stimulated (lanes 2, 4, and 6) TF-1 cells were immunoprecipitated (IP) with anti-Shc (lanes 1 and 2) or anti-Grb2 (lanes 3 and 4) antibodies or adsorbed with a GST fusion protein containing the SH2 domain of Grb2 (Grb2 SH2; lanes 5 and 6). The precipitates were analyzed by SDS-PAGE followed by antiphosphotyrosine immunoblotting (lanes 1 through 6). Arrows indicate the position of the detected phosphotyrosyl proteins and the GST-Grb2-SH2 fusion protein. (B) Binding of Grb2 to tyrosine-phosphorylated p140 is mediated through the SH3 domains of Grb2. Cell lysates of unstimulated (lanes 1, 3, 5, and 7) or hGM-CSF–stimulated (lanes 2, 4, 6, and 8) TF-1 cells were immunoprecipitated with anti-Shc antibodies (lanes 1 and 2) or adsorbed with either a GST fusion protein containing the C-terminal SH3 domain of Grb2 (GST Grb2 SH3-C; lanes 3 and 4), the N-terminal SH3 domain of Grb2 (GST Grb2 SH3-N; lanes 5 and 6), or with GST vector protein (GST) (lanes 7 and 8). The precipitates were analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 8).
During these experiments, we detected a tyrosine-phosphorylated protein of 140 kD in anti-Grb2 immunoprecipitates from hGM-CSF–stimulated cells (Fig 7A, lane 4), which comigrated with p140 detected in anti-Shc immunoprecipitates (Fig 7A, lane 2). As shown in lane 6 of Fig 7A, tyrosine-phosphorylated p140 did not associate with the SH2 domain of Grb2 but associated with the C-terminal and more weakly with the N-terminal SH3 domain of Grb2 (Fig 7B, lanes 4 and 6, respectively). Therefore, in TF-1 cells, hGM-CSF induced the association of phosphotyrosyl p140 with Grb2, and this association was mediated by the Grb2 SH3 domains. Further analysis is required to determine if p140 binds simultaneously to Shc and Grb2 or whether separate p140/Shc and p140/Grb2 complexes are formed in response to hGM-CSF.
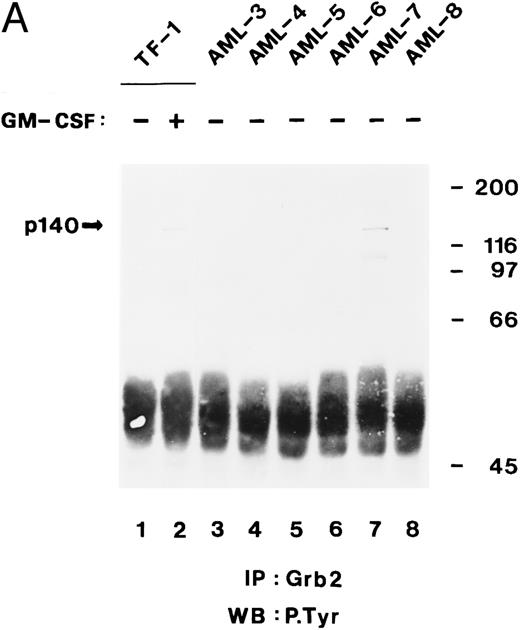
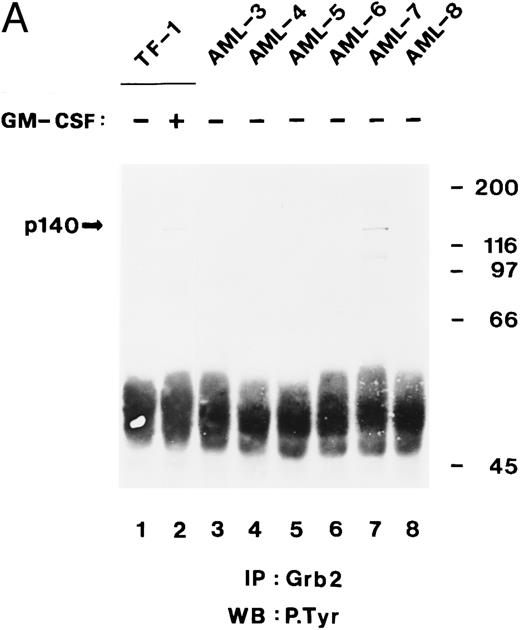
Tyrosine-phosphorylated p140 binds to the C-terminal SH3 domain of Grb2 in some AML cells.In the previous sections, we showed that tyrosine-phosphorylated p140 was constitutively associated with Shc through the PTB domain in three of five AML analyzed (Fig 5B) and that p140 associated with Grb2 through its SH3 domains. We therefore analyzed whether in AML p140 was also constitutively associated with Grb2 and whether this association was mediated through the SH3 domain of Grb2. Cell lysates from unstimulated and hGM-CSF–stimulated TF-1 cells and from six AML (AML-3 through AML-8) were immunoprecipitated with anti-Grb2 antibodies and analyzed by antiphosphotyrosine immunoblotting. As shown in Fig 8A, a tyrosine-phosphorylated protein of 140 kD coprecipitated with Grb2 in hGM-CSF–stimulated TF-1 cells and in AML-7. These data indicate that in AML-7, Grb2 and p140 were constitutively associated in vivo.
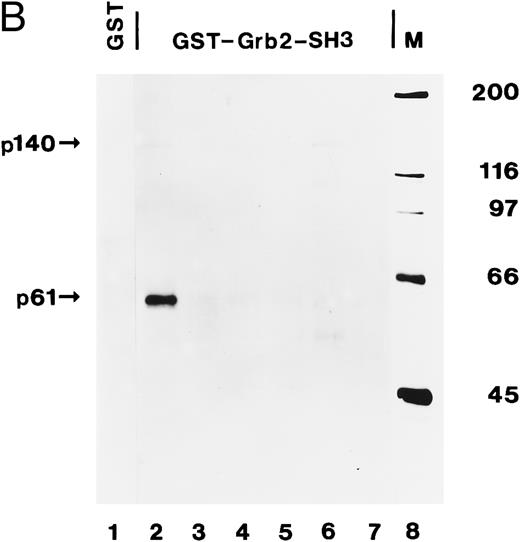
The C-terminal SH3 domain of Grb2 binds to tyrosine-phosphorylated p140 in AML. (A) p140 is constitutively associated with Grb2 in AML cells. Cell lysates from unstimulated (lane 1) or hGM-CSF–stimulated TF-1 cells (lane 2) and from unstimulated primary leukemia cells obtained from five patients with AML (AML-3 through AML-8; lanes 3 through 8) were immunoprecipitated with anti-Grb2 antibodies followed by antiphosphotyrosine immunoblotting (P.Tyr). The position of p140 is indicated on the left. (B) The C-terminal SH3 domain of Grb2 binds to p140 in AML cells. Cell lysates from unstimulated primary AML cells were incubated with either GST vector protein (GST; AML-3; lane 1) or with GST fusion proteins containing the C-terminal SH3 domain of Grb2 (GST-Grb2-SH3; AML-3 through AML-8; lanes 2 through 7). Associated tyrosine-phosphorylated proteins were analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 7). The AML samples analyzed in this experiment correspond to the AML-3 through AML-8 samples analyzed in (A), lanes 3 through 8, and in Fig 4B, lanes 2 through 7. The position of tyrosine-phosphorylated proteins is indicated on the left. Molecular weight markers are indicated on the right.
The C-terminal SH3 domain of Grb2 binds to tyrosine-phosphorylated p140 in AML. (A) p140 is constitutively associated with Grb2 in AML cells. Cell lysates from unstimulated (lane 1) or hGM-CSF–stimulated TF-1 cells (lane 2) and from unstimulated primary leukemia cells obtained from five patients with AML (AML-3 through AML-8; lanes 3 through 8) were immunoprecipitated with anti-Grb2 antibodies followed by antiphosphotyrosine immunoblotting (P.Tyr). The position of p140 is indicated on the left. (B) The C-terminal SH3 domain of Grb2 binds to p140 in AML cells. Cell lysates from unstimulated primary AML cells were incubated with either GST vector protein (GST; AML-3; lane 1) or with GST fusion proteins containing the C-terminal SH3 domain of Grb2 (GST-Grb2-SH3; AML-3 through AML-8; lanes 2 through 7). Associated tyrosine-phosphorylated proteins were analyzed by antiphosphotyrosine immunoblotting (lanes 1 through 7). The AML samples analyzed in this experiment correspond to the AML-3 through AML-8 samples analyzed in (A), lanes 3 through 8, and in Fig 4B, lanes 2 through 7. The position of tyrosine-phosphorylated proteins is indicated on the left. Molecular weight markers are indicated on the right.
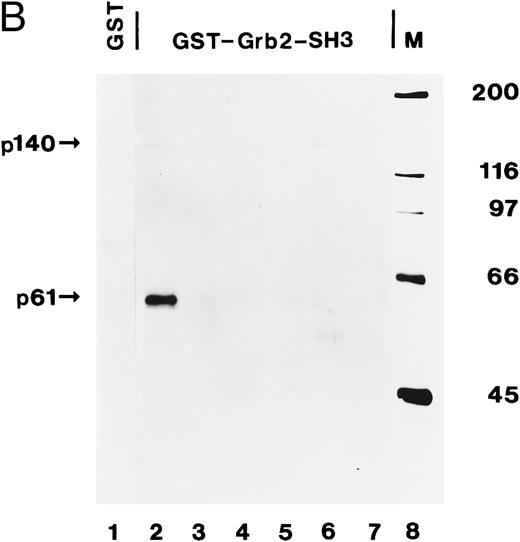
We then incubated lysates from the same AML (AML-3 through AML-8) with the C-terminal SH3 domain of Grb2 expressed as a GST fusion protein. After adsorption on glutathione agarose and antiphosphotyrosine immunoblotting, p140 was detected in two of the six AML analyzed, ie, AML-3 and AML-7 (Fig 8B, lanes 2 and 6). In the case of AML-3, we were not able to detect p140 in anti-Grb2 immunoprecipitates (Fig 8A, lane 3), suggesting that the amount of p140 that can be purified with a bacterially expressed Grb2 fusion protein is higher than by coimmunoprecipitation with anti-Grb2 antibodies. An additional tyrosine-phosphorylated protein of 61 kD was also recognized by the SH3 domain of Grb2 in AML-3 cells (Fig 8B, lane 2), which may be the same 61-kD protein that was present in anti-Shc immunoprecipitates from AML-3 (Fig 4B, lane 2).
DISCUSSION
The identification of constitutively activated signalling pathways involved in transducing an oncogenic signal in leukemia cells is likely to shed light on the mechanisms of leukemogenesis and may show new targets for antileukemia therapy. In this study, we have focused on the analysis of two adaptor proteins, Shc and Grb2, that are both involved in the transduction of signals from a variety of activated receptors to downstream targets. The experiments reported here show that (1) Shc is constitutively phosphorylated on tyrosine in all AML analyzed; (2) a 140-kD tyrosine-phosphorylated protein (p140) is constitutively associated with Shc and Grb2 in some cases of AML in vivo; (3) the association of p140 with Shc and Grb2 is mediated through the PTB domain of Shc and the SH3 domains of Grb2, respectively; and (4) additional tyrosine-phosphorylated proteins of 61 kD and 200 kD become part of the Shc/Grb2 signalling complex in some AML.
Shc proteins become rapidly tyrosine-phosphorylated upon stimulation of receptor tyrosine kinases such as the EGF receptor44 after stimulation of receptors without intrinsic tyrosine kinase activity, such as the EPO receptor, IL-3 receptor, and GM-CSF receptor,55,56,68 and by activated tyrosine kinases such as v-Src or p210Bcr/Abl.54,55 In normal unstimulated PBMCs, we did not detect any tyrosine phosphorylation of Shc, indicating that Shc is not activated in these cells. The same was true for a factor-dependent erythroleukemia cell line (TF-1), in which no Shc phosphorylation was detectable after starvation of the cells in factor- and serum-deprived medium. Stimulation of TF-1 cells with hGM-CSF resulted in a transient phosphorylation of Shc that returned to baseline levels after stimulation, indicating that, in this factor-dependent leukemia cell line, the activation and inactivation of Shc can still be regulated. However, in freshly isolated samples from AML, Shc proteins were found to be constitutively phosphorylated on tyrosine in all cases analyzed. This is in agreement with a recent report that the constitutive phosphorylation of Shc proteins occurs frequently in human tumors including acute leukemias.69 These data indicate that Shc is activated in AML cells and may be involved in the constitutive stimulation of a signalling pathway. This signalling pathway may be the Ras pathway as discussed below in more detail.
During this analysis we identified three different tyrosine-phosphorylated proteins of 200, 140, and 61 kD that formed a constitutive complex with Shc in AML cells. Constitutive binding of the 140-kD protein (p140) to Shc was detected in four of the eight AML analyzed. Comparison of the French-American-British (FAB) class and karyotype of the four AML showing constitutive association of Shc with p140 did not show a correlation between the appearance of the Shc/p140 complex and either the FAB class or the chromosomal abnormalities observed (data not shown). A similar or identical 140-kD phosphoprotein was found to associate with Shc transiently after stimulation of factor-dependent cell lines with hGM-CSF or CSF-1, indicating that p140 is involved in signalling by different types of receptors, ie, cytokine receptors without intrinsic tyrosine kinase activity and receptor tyrosine kinases. Binding of Shc to a tyrosine-phosphorylated protein of 140-150 kD that might be identical with our p140 protein has also been detected after stimulation of cells with a variety of growth factors and cytokines, suggesting that the association of p140 to Shc is a common event upon receptor stimulation.53,55,57,70 71
p140 was not recognized by antibodies directed against known signalling proteins in the same molecular weight range, suggesting that p140 was a novel signalling protein. To further characterize this protein, we analyzed the nature of its interaction with Shc and with Grb2. Although the SH2 domain of Shc can bind to phosphotyrosine sites in activated receptors and other proteins, the SH2 domain of Shc was not involved in its association with phosphorylated p140 (data not shown). Using an MBP-N-Shc fusion protein, we showed that the ligand-dependent binding of p140 to Shc in factor-dependent cell lines and the constitutive binding of p140 to Shc in AML cells was mediated instead by the PTB domain of Shc. Because the PTB domain of Shc binds to an NPXpY motif in several tyrosine phosphorylated proteins, we expect that the p140 protein contains an NPXpY motif. Recently, a protein of 145 kD that associates with the PTB domain of Shc after stimulation with several cytokines has been cloned and named SHIP for SH2 containing inositol phosphatase.72,73 This protein contains two NPXpY motifs and also binds to the C-terminal SH3 domain of Grb2.72 Whether SHIP is identical with our p140 protein has to be shown in further experiments.
In hGM-CSF–stimulated TF-1 cells, tyrosine-phosphorylated Shc associated with the SH2 domain of Grb2, as has been shown in other systems.37 During this analysis, we detected a 140-kD tyrosine-phosphorylated protein in anti-Grb2 immunoprecipitates that comigrated with the 140-kD protein detected in anti-Shc immunoprecipitates, suggesting that they are identical. Binding of the 140-kD protein to Grb2 was mediated through both SH3 domains of Grb2. A protein of 140 kD that coprecipitates with Shc and binds to the SH3 domains of Grb2 before and after stimulation with hGM-CSF has been described by Lanfrancone et al.53 However, in our experiments, tyrosine-phosphorylated p140 was detectable in a complex with Grb2 after stimulation with hGM-CSF, but not in unstimulated cells. Although the association of phosphorylated p140 with the SH3 domains of Grb2 in TF-1 cells was dependent on hGM-CSF stimulation, phosphorylated p140 was constitutively associated with the SH3 domain of Grb2 in two of six AML analyzed. Interestingly, these were the same two AML in which p140 was also constitutively bound to the PTB domain of Shc. These data suggest that once p140 is phosphorylated, it becomes associated with Shc and Grb2 and that these interactions can occur either transiently in factor-dependent cell lines or constitutively in AML cells.
Three major questions about the possible meaning of the activated Shc complexes in AML cells arise from our results. First, which are the receptors and/or cytoplasmic tyrosine kinases that are involved in phosphorylating Shc? Secondly, which signalling pathway(s) are triggered by the activated Shc complex? Third, does the constitutively activated Shc complex have a transforming capacity in myeloid cells that may contribute to the pathophysiology of AML?
Regarding the identification of the tyrosine kinase that phosphorylates Shc, it has been recently shown that Shc proteins are common substrates of several constitutively activated tyrosine kinases in human tumors.69 Recently, we have detected tyrosine-phosphorylated Shc and p140 in immunoprecipitates of Src family members, ie, Src/Yes and Lyn in hGM-CSF–stimulated TF-1 cells.64,74 Thus, our data suggest that members of the Src family of tyrosine kinases may be involved in the phosphorylation of Shc and/or p140. The identification of Shc as a Src-SH3 domain binding protein75 is consistent with the idea that Shc may be a direct substrate of Src family member(s) in human AML.
An important question is which signalling pathway(s) are triggered in AML by the activated Shc complexes. A large body of evidence implicates Shc in activation of the Ras signalling pathway, which plays a central role in relaying downstream signals from activated receptors on the cell surface, or from activated tyrosine kinases to transcription factors in the nucleus.35,76,77 It has been shown that phosphorylated Shc proteins bind the Grb2-SOS complex that leads to activation of Ras37,41 and that the formation of Shc-Grb2 complexes is necessary to induce transformation of immortalized fibroblasts.78 In addition, overexpression of Shc proteins increased the ligand-dependent activation of the Ras signalling pathway,53 and a dominant negative mutant of Shc can inhibit the CD4/Lck-mediated activation of Ras.79 It seems therefore possible that the constitutively activated Shc complexes in AML are involved in activation of the Ras signalling pathway and that this mechanism of Ras activation may contribute to the pathophysiology of AML. In preliminary experiments, we have detected activated Raf and MAP kinases in one case of AML with a constitutive Shc/Grb2/p140 complex and in another case with a constitutive Shc/p200 complex, whereas Raf and MAP kinase activation was not detected in four other cases with activated Shc proteins (M.J. and R.A.F., unpublished results). These data suggest that the activated Shc complexes may trigger the Ras signalling pathway in some cases of AML, whereas in other cases, Shc may activate other signalling pathway(s) that have not been identified so far. Whether the constitutively activated Shc complexes have the capacity to transform myeloid cells and play a role in the pathophysiology of AML will require further analysis.
ACKNOWLEDGMENT
We thank J.C. Gutheil for generously providing some of the leukemia samples used in this study. We are grateful to M.A. Tate for excellent technical assistance. We thank A. Miyajima for providing TF-1 cells, MoAb directed against the β subunit of hGMR, and recombinant human GM-CSF; J. Schlessinger, E. Lowenstein, and A. Batzer for the anti-Grb2 MoAb EL6 and the Grb2 SH2 domain; L. Lanfrancone and P.G. Pelicci for the SH2 domain of Shc; T. Gustafson for the N-terminal domain of Shc; A.M. Pendergast for the SH3 domains of Grb2; and E.R. Stanley for recombinant CSF-1.
Supported by National Cancer Institute Grant No. R29 CA 55293, by the National Leukemia Association, and by UMAB DRIF award to R.A.F. M.J. was a recipient of a Deutsche Forschungsgemeinschaft fellowship from the Federal Republic of Germany.
Address reprint requests to Ricardo A. Feldman, PhD, University of Maryland School of Medicine, Department of Microbiology and Immunology, BRB 13-043, 655 W Baltimore St, Baltimore, MD 21201.