Abstract
Immune-mediated effects appear to play a major role in controlling minimal residual disease (MRD). We, therefore, investigated the role of recombinant human interleukin-2 (rIL-2) given concomitantly with interferon-α (IFN-α) in malignant lymphoma (ML) patients with responding disease following autologous bone marrow or blood stem cell transplantation (ABSCT). Fifty-six patients were included in this investigation. Thirty-two patients had non-Hodgkin's lymphoma (NHL) and 24 patients had Hodgkin's disease (HD). Sixty-one patients (NHL 36, HD 25) served as historical controls. Patients from both groups had similar demographic characteristics, the same stage of disease at presentation, status of disease at transplantation, conditioning regimens, and type of transplant. rIL-2 and IFN-α were selfadministered in two cycles beginning 2.5 to 10.5 months (median, 4 months) posttransplant and separated by a 4-week interval. Each cycle consisted of IFN-α subcutaneously (SC) 3 × 106 U/d × 5 d/wk combined with rIL-2 SC 3 to 6 IU/m2/d × 5 d/wk for 4 weeks. The incidence of survival and disease-free survival (DFS) was significantly higher in the group under investigation than in the historical controls (P < .01). Of 56 patients with ML treated with IFN-α + rIL-2, 45 patients are DFS (80.4%) after a follow-up of 7 to 78 months (median, 34 months), whereas in the historical controls, 32 of 61 (52.5%) patients are disease free, in a follow-up of 4 to 84 months (median, 23 months) posttransplant (P < .01). Our preliminary results are encouraging and suggest that home administered immunotherapy with IFN-α and rIL-2 is relatively well tolerated and may intensify remission in ML patients with MRD following ABSCT.
HIGH-DOSE CHEMOTHERAPY in conjunction with autologous bone marrow or blood stem cell transplantation (ABSCT) is increasingly being used for the treatment of patients with non-Hodgkin's lymphoma (NHL) and Hodgkin's disease (HD).1-12 There are data indicating that this treatment regimen may improve long-term disease-free survival (DFS) in several categories of patients with malignant lymphoma (ML).3,6,7,12 However, the relapse rate still remains high.1-12 Residual tumor cells resistant to high-dose chemoradiotherapy may be responsible for the still relatively high rate of disease recurrence following this procedure. It is believed that the high relapse rate of disease following ABSCT is due to the lack of immune response of immunocompetent cells against residual tumor cells.13-17
Recently it has been demonstrated that immune-mediated graft versus lymphoma similarly to graft versus leukemia effect may be induced in certain categories of NHL recipients of allogeneic marrow grafts.6,18-20 This suggests that one of the ways to reduce relapse rates post-ABSCT may be to intensify immune-mediated effector mechanisms against residual tumor cells.21,22 A number of cytokines have demonstrated antitumor activity in lymphoma patients. One of these is interferon-α (IFN-α), which when administered by itself or in conjunction with chemotherapy, has been shown to be effective in evoking antitumor response in 40% to 50% of the patients with low-grade NHL studied, including complete response (CR) in 5% to 10% of these patients.23-25 The results of IFN-α given together with chemotherapy have proven significantly better than chemotherapy alone.26
Recombinant interleukin (rIL-2) is another novel activator of natural killer (NK) cells and T lymphocytes with cytotoxic capacities27,28 that has been shown to have an antitumor effect in several animal models25-30 including recipients of syngeneic bone marrow transplant29,30 and in pilot clinical trials on patients with NHL and HD.31-37 This cytokine has achieved encouraging preliminary results including some complete CRs over a follow-up period of up to 26 months.35 Combination therapy models of rIL-2 and IFN have been demonstrated to augment NK cytolytic activity both in vitro and in vivo in mice and humans.36-44 In view of the above and to reduce relapse rates following autologous transplant, rIL-2 has been administrated in pilot clinical trials to lymphoma patients post-ABSCT with encouraging results.45-49 We have conducted a phase IIb clinical trial on 56 ML patients with minimal residual disease (MRD) post-ABSCT, treated with a combination of rIL-2 and IFN-α subcutaneously (SC), in an outpatient setting and compared the results with 61 matched historical controls.
MATERIALS AND METHODS
Patients.Fifty-six patients (36 men, 20 women), median age, 35 (10 to 53) years were enrolled in the study. Thirty-two patients had NHL and 24 patients had HD. Sixty-one ML patients (NHL 36, HD 25) served as historical controls (Table 1). Disease stage, sex, and age were statistically similar in the study group and the historical controls: 44 patients (79%) in the study group and 43 patients (70%) in the historical controls were stage III-IV at diagnosis, while 12 patients (21%) in the study group and 18 patients (30%) in the historical controls were stage I-II (Table 2). Thirty-three patients (58%) in the study group and 37 patients (60%) in the historical control group had B symptoms (Table 2). The frequency of NHL high-grade histology was 36% in the controls in comparison with 22% in the study group. Most of the patients in the study group and historical controls were transplanted in an advanced stage of disease, as 63% of the study group patients and 69% of the historical controls were transplanted after first relapse or more (Table 2). Conditioning regimen (TECAM) included thiotepa (40 mg/m2 × 4 days), etoposide (200 mg/m2 × 4 days), cytosar (200 mg/m2 × 4 days), cyclophosphamide (60 mg/kg × 1 day), and melphalan (60 mg/m2 × 2 days). There was no difference in the conditioning regimen between the immunotherapy-treated patients and the historical control group. Twenty-four hours after the last chemotherapy treatment, the patients received nonpurged cryopreserved autologous marrow or peripheral blood stem cells collected with a Cs 3000plus (Baxter Healthcare Corp, Deerfield, IL) following 5 days of treatment with 10 μg/kg granulocyte colony-stimulating factor (neupogen).
The median time interval between ABCST and cell-mediated immunotherapy (CMI) was fixed at 4 (2.5 to 10) months, in view of the need for adequate hematopoietic reconstitution before the initiation of cytokine therapy. Only patients without disease progression for 4 months posttransplant were considered eligible for the CMI protocol. Hence, patients in the historical control group with disease progression during the 4 months post-ABSCT were also excluded from further analysis. None of the patients were intentionally excluded from the CMI protocol. Refusal to enter an experimental protocol, technical difficulties in obtaining cytokines, mainly rIL-2, due to inadequate supply, were among the reasons for noninclusion in the study.
Study parameters.Before the initiation of immunotherapy, all patients had their complete medical history taken and underwent physical examination, chest x-ray, electrocardiogram, prothrombin time (PT), partial thromboplastin time (PTT), complete blood count, and biochemistry tests. Patients were examined once weekly. Hematologic tests (complete blood counts including the differential, as well as biochemistry tests) were performed once weekly. Patients were monitored for adverse effects and drug toxicity. Survival, DFS, and relapse rates were assessed.
Treatment schedule.After stabilization of peripheral blood counts (white blood cell >2.5 × 109/L and platelets >75 × 109/L), patients were treated with daily SC injections of Chiron rIL-2 (Proleukin) (3 to 6 × 106 international units (IU)/m2/d), combined with IFN-α (Roferon A; Hoffman La Roche, Switzerland) 3 × 106 U/d, for 5 consecutive days each week for 4 weeks. The median time from ABSCT until the onset of immunotherapy was 4 (2.5 to 10.5) months. Patients received two identical cycles of 4 weeks of immunotherapy consisting of a combination of rIL-2 and IFN-α followed by a 4-week rest period (a total of 40 injections given over a 3-month period). Toxicities were evaluated according to the World Health Organization (WHO) toxicity grading scale.
Supportive care.Patients received paracetamol (500 mg/6 hours) as prophylaxis and therapy for controlling fever, myalgia, and muscle stiffness. Promethazine (25 mg/8 hours) was prescribed to control chills and allergic reaction and ondansetron hydrochloric dihydrate (Zofran) (8 mg × 3 days) or Navoben (5 mg × 1 day) to control nausea and vomiting. For other adverse events, treatment was given according to clinical symptoms.
rIL-2 (Proleukin).Human rIL2 was kindly provided by Chiron BV, Amsterdam, The Netherlands. Specific activity was approximately 1.5 × 107 U/mg protein. αIL-2 was supplied as a lyophilized powder in 1-mg vials (18 × 106 IU) and reconstituted in sterile water. IU are used throughout the report.
IFN-α (Roferon-A).Recombinant human IFN-α (Roferon A) was provided by Hoffmann La Roche. Specific activity was 2 × 108 U/mg protein. It was supplied as a lyophilized powder and reconstituted in sterile water.
Statistical evaluation.The clinical and demographic characteristics of ML, HD, and NHL patient groups who received immunotherapy and patients belonging to the historical control group who did not receive immunotherapy were analyzed with “Fisher-Irwin exact test.”
A multivarianate Cox proportional hazards analysis model was used to analyze improved DFS in patients who received or did not receive immunotherapy and other risk factors.50
The Kaplan-Meier (KM) method was used to calculate the probability of survival and DFS as a function of time in the ML, HD, and NHL groups of patients with and without immunotherapy.51
The “Log Rank test” was used to compare between pairs of KM curves.52
RESULTS
We compared the clinical outcome of patients receiving cytokine-mediated immunotherapy (CMI) post-ABSCT with that of patients who did not receive CMI post-ABSCT for the same malignancies in our institution. In particular, there were no significant differences among the groups with respect to patient sex, diagnosis, and stage of disease at BMT. There was significant difference among the groups in age, the NHL historical controls were significantly younger (P < .02) (Table 1). There was no difference in ablative regimen between the groups. In addition, risk factors associated with improved DFS post-ABSCT in patients who received immunotherapy, were analyzed with the multivariant Cox proportional hazards analysis model. The independent variables that were included in the analysis were: age, sex, systemic symptoms, and stage of disease; disease status at BMT showed that of all the independent variables analyzed above, only age was found to be significant in the development of relapse, with a relative risk of 1.14 (P < .02) (ie, older patients tend to relapse more than younger patients) (Table 2). Other than this, we found no candidate variables among patient characteristics or clinical disease factors that differed between the study group and the control group.
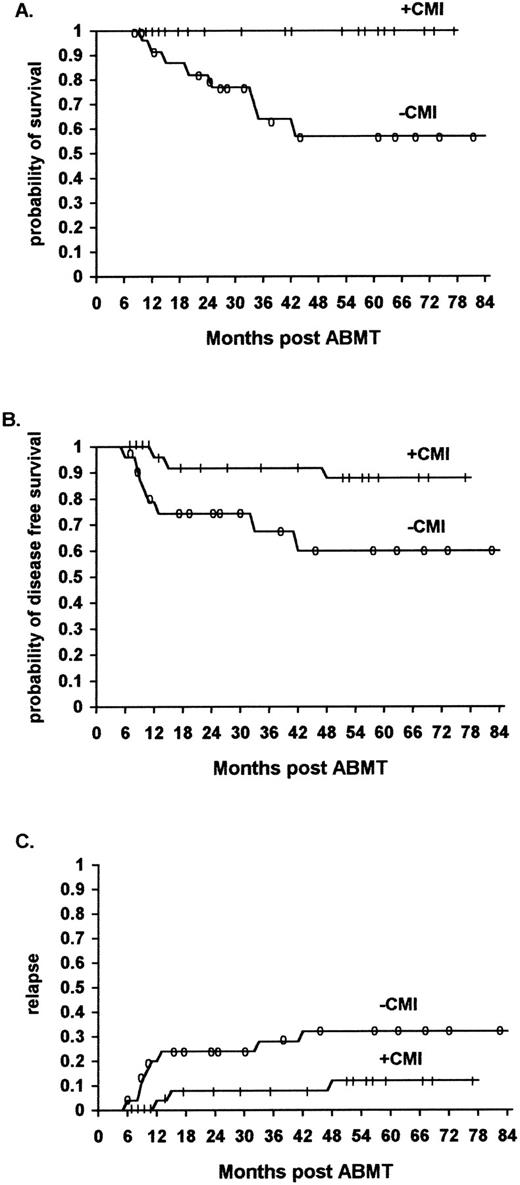
Survival.The overall survival of ML patients who received immunotherapy was significantly higher than that of ML patients not receiving immunotherapy. The survival at 48 months was 90% (95% confidence interval 70% to 97%) for the immunotherapy patients, and 46% (95% confidence interval 30% to 60%) for the historical controls (P < .01) (Fig 1A) (Table 3).
The effect of IL2/IFN-α immunotherapy on actuarial survival (A), DFS (B), and relapse (C) in ML patients who received immunotherapy (n = 56) versus historical controls (n = 61, TRT + R) after autologous bone marrow transplantation (P < .01, P < .01, and P < .01, respectively).
The effect of IL2/IFN-α immunotherapy on actuarial survival (A), DFS (B), and relapse (C) in ML patients who received immunotherapy (n = 56) versus historical controls (n = 61, TRT + R) after autologous bone marrow transplantation (P < .01, P < .01, and P < .01, respectively).
Similarly, the overall survival was significantly higher for the HD and NHL patients who received immunotherapy as compared with the historical controls. The survival rates at 48 months were 100% and 80% (95% confidence interval 43% to 95%) versus 57% (95% confidence interval 31% to 76%) and 42% (95% confidence interval 24% to 58%), respectively (P < .02) (Fig 2A, P < .01, and Fig 3A, P < .02) (Table 3).
The effect of rIL-2/IFN-α immunotherapy on actuarial survival (A), DFS (B), and relapse (C) in NHL patients who received immunotherapy (n = 32) versus controls (n = 36, TRT + R) after autologous bone marrow transplantation (P < .01, P < .01, and P < .01, respectively).
The effect of rIL-2/IFN-α immunotherapy on actuarial survival (A), DFS (B), and relapse (C) in NHL patients who received immunotherapy (n = 32) versus controls (n = 36, TRT + R) after autologous bone marrow transplantation (P < .01, P < .01, and P < .01, respectively).
The effect of rIL-2/IFN-α immunotherapy on acturial survival (A), DFS (B), and relapse (C) in Hodgkin's patients who received immunotherapy (n = 24) versus historical controls (n = 25, TRT + R) after autologous bone marrow transplantation (P < .02, P < .042, and NS, respectively).
The effect of rIL-2/IFN-α immunotherapy on acturial survival (A), DFS (B), and relapse (C) in Hodgkin's patients who received immunotherapy (n = 24) versus historical controls (n = 25, TRT + R) after autologous bone marrow transplantation (P < .02, P < .042, and NS, respectively).
DFS.The overall DFS of ML patients who received immunotherapy was significantly higher than that of comparable ML patients in the historical control group who did not receive immunotherapy. The actuarial DFS at 48 months was 70% (95% confidence interval 50% to 84%) and 48% (95% confidence interval 32% to 61%), respectively (P < .01) (Fig 1B) (Table 3).
Similarly, the actuarial DFS was significantly higher for the NHL and HD patients after immunotherapy as compared with the historical controls. The actuarial DFS with NHL receiving immunotherapy at 48 months was 64% (95% confidence interval 36% to 80%) and 41% (95% confidence interval 25% to 48%) for patients who did not receive immunotherapy (P < .01) (Fig 2B). The actuarial DFS of patients with HD receiving immunotherapy at 48 months was 88% (95% confidence interval 50% to 96%) and 60% (95% confidence interval 34% to 78%) for patients who did not receive immunotherapy (P < .042) (Fig 3B) (Table 3).
The relapse rate was significantly lower for ML patients who received immunotherapy in comparison to a similar cohort of patients belonging to the historical controls. Of the 56 patients who received immunotherapy, 11 (20%) relapsed (8 NHL and 3 HD patients), while of the 61 patients who did not receive immunotherapy 29 (46%) relapsed (21 NHL and 8 HD patients) (P < .01) (Fig 1C, P < .01, Fig 2C, P < .01, Fig 3C, not significant [NS] ).
Toxicity.All patients were evaluated for treatment-related adverse events. Most of the toxic effects improved gradually with therapy and were manageable with standard antipyretic and analgesic agents. Most of the patients continued their regular activities while receiving treatment. WHO grade II-III fever, chills, and fatigue were very common and occurred in 86% of the patients. The intensity of fever, chills, and fatigue tended to decrease throughout the treatment course (fewer patients experienced these side effects on the second cycle of therapy than on the first). Anorexia (46.5%), nausea with or without vomiting (77%), and diarrhea were common adverse events. A mild erythematous, pruritic maculopapular rash was observed in 34% of the patients. Three patients had grade II-III hair loss. Mild neurotoxicity, consisting of depression, insomnia, and nervousness was observed in 34% of the patients. Mild to moderate anemia (8 to 11 g%) was observed in 50% of the patients, while mild to severe thrombocytopenia without bleeding tendency occurred in 80% of the patients. Only nine patients required red blood cell or platelet transfusions. Grade II-III liver enzyme elevation was observed in half of the patients. The SC administration of IFN-α and rIL-2 resulted in transient inflammation and local induration of the injection sites, which persisted for up to 2 weeks after treatment. Allergic manifestations, including contact dermatitis53 and bronchial asthma (one patient), were observed. One patient developed severe cardiotoxicity with cardiogenic shock responding to intravenous fluid and dopamine support.54 55 All side effects improved gradually and resolved after termination of the treatment. In only two cases did treatment have to be discontinued or interrupted because of toxicity (Table 4).
DISCUSSION
This report describes results of a nonrandomized out-patient treatment program based on administration of IFN-α and rIL-2 for the treatment of MRD in patients with lymphoma post-ABSCT. Combined IFN-α and rIL-2 immunotherapy resulted in a significant enhancement of survival and DFS as a result of reduced relapse rates in both NHL and HD patients post-ABSCT, in comparison to historical controls. Of 56 patients with HD and NHL treated with CMI, 45 patients are in CR (80.4%), after a follow-up of 7 to 78 months (median, 34 months). In comparison, of the 61 historical control patients, 32 are in CR (52.5%), after a follow-up of 4 to 84 (median, 23) months.
Prevention of relapse postautologous transplant may be achieved either by intensifying pretransplant chemoradiotherapy which, however, has been shown to augment organ toxicity and predisposition to early transplant-related death, or by intensifying the patient's immunocompetent effector cells against residual tumor cells by cytokine administration.36 There is evidence that tumor cell lines that have become resistant to chemotherapeutic agents are still susceptible to lysis by rIL-2 activated killer cells.56 Moreover, the synergism of IFN-α and rIL-2 shown to be effective in our murine model of lymphoid leukemia/lymphoma (unpublished data), as well as in other tumors in experimental animals and man,43 46 may increase the antitumor effect by various mechanisms.
While IFN-α has a direct antiproliferative cytoreductive effect, it may also help amplify the antitumor effects of the host's immune cells by augmentation of the expression of class I and class II cell-surface molecules.57 rIL-2 manifests its antitumor effects through stimulation of T-cell–dependent and mostly NK cell-dependent immune reactivity, as has been documented in vitro in man and in vivo in murine leukemia.27,28,39-42,47,48 Both IFN-α and rIL-2 have been previously administered to patients with malignant lymphoma with encouraging results.23-26,31-35 rIL-2 was previously shown by us to be effective in the setting of MRD30 particularly following syngeneic BMT,23 in analogy with ABSCT in man.
Abnormal T-cell parameters have been observed to linger following autologous transplant, which may account for the immunodeficiency observed up to a year or more posttransplant. Quantifiably, abnormal T-cell parameters following ABMT include a depressed absolute number of CD4+ T cells, a decreased response to mitogens, antigens, or allogeneic cells in lymphoproliferative assays, decrease in vitro T-cell colony formation, and a profound impairment of IL-2 production.58-62 Peripheral blood NK activity, although rapidly restored to pretransplant values, remains lower than normal control values.62
Because endogenous IL-2 production is decreased for about 1 year following ABSCT, administration of exogenous rIL-2 to these patients might be expected to enhance immune reconstitution and reduce the risk of relapse. Accordingly, rIL-2 has been administered recently postautologous transplant to leukemic patients in an attempt to provide an antileukemic effect, demonstrating some response in acute myeloid leukemia (AML) patients.63 Interestingly, no such positive data have been reported for patients with acute lymphoblastic leukemia (ALL).64 Fefer et al44,45 administered rIL-2 + lymphokine activated killer (LAK) cell therapy soon after ABMT to 16 high-risk ML patients (NHL, 12; HD, 4). Of these patients, five have relapsed, while 11 remain in complete remission 6 to 21 (median, 10) months post-ABMT.44,45 Others have demonstrated similar results with continuous high-dose rIL-2 administrated to ML patients resulting in in vitro reconstitution of NK and T-cell functions.65-67
LAK precursor activity has been found to reconstitute rapidly after ABSCT in lymphoma patients. This may provide the biological rationale for rIL-2 consolidative immunotherapy post-ABSCT for ML patients.68 Our immunological evaluation of patients with hematologic malignancies treated with IFN-α + rIL-2 SC following ABSCT, demonstrated that cytokine therapy could stimulate the host immune system by amplifying cytotoxic activity of K562 and Daudi cell lines, as well as T-cell–dependent mitogenic response.36 Similarly, Higuchi et al69 demonstrated that in lymphoma patients who received high-dose rIL-2 post-ABMT, a higher percentage of the NK cell markers CD16 and CD56 was found in blood cells. Augmented lysis of K562 and Daudi cell lines was also found, which may reflect the induction of NK and circulating LAK effector activities.
Bosly et al70 demonstrated an increase of CD8 T cells and CD56+ NK cells and the correction of functional T-cell defects in lymphoma patients who received rIL-2 post-ABMT. IFN-α has also been shown to intensify NK cell-mediated cytotoxicity27,35 and to have a therapeutic effect in lymphoma patients, either alone or in combination with chemotherapy.23-26,71 Because rIL-2 and IFN-α have demonstrated their antitumor effect when administered alone,25,26,31-35 we took advantage of their synergistic effect to use them in combination. The rationale behind this combination was that IFN-α, in addition to providing a direct antiproliferative effect, may also be able to enhance the expression of specific antigens on target cells. These could then be more efficiently recognized and lysed by T cells activated and expanded by rIL-2. Vivancos et al72 have also recently administered IFN-α and rIL-2 in combination at relatively high doses to 16 patients (mostly leukemic) post-ABMT with tolerable toxicity.
Combination cytokine therapy with IFN-α and rIL-2 has been explored in solid tumor malignancies.73-77 The rationale for combination therapy with IFN-α and IL-2 derived from both in vitro and in vivo observation.78 Out-patient combination cytokine therapy has been successfully used in patients with solid tumor malignancy. Based on this clinical data, we administered IFN-α and rIL-2 combined, SC, on an out-patient basis to 56 ML patients 2.5 to 10 (median, 4) months post-ABSCT with very encouraging results. Combined ambulatory CMI with IFN-α and r-IL2 was found to be feasible and safe, with tolerable side effects.
Side effects were those expected from IFN-α and rIL-2 when given as single agents, or recently in combination for patients with other malignancies.73-77 They included fever, chills, fatigue, flu-like symptoms, anorexia, nausea, vomiting, and diarrhea and were transient and reversible. Most of the patients were able to continue their regular activities with the help of symptomatic treatment. The overall side effects of the combined treatment modality were less toxic than those published on intravenous administration of high-dose rIL-2 only.65
In summary, our study on lymphoma patients demonstrated that combined IFN-α and rIL-2 immunotherapy at the stage of MRD post-ABSCT intensifies remission and reduces relapse rates in comparison to historical controls. Cytokine-mediated immunotherapy activates the cytologic activity of the host immune system, thus mediating possible antitumor response, which may result in increased survival and DFS in comparison to historical controls. Prospective randomized studies are being planned to confirm our encouraging results.
ACKNOWLEDGMENT
The authors thank Prof A. Polliack, Prof E. Rachmilewitz, and all of the physicians for referring patients who were included in this study.
Address reprint requests to A. Nagler, MD, MSc, Bone Marrow Transplantation Department, Hadassah University Hospital, POB 12000, Jerusalem 91120, Israel.