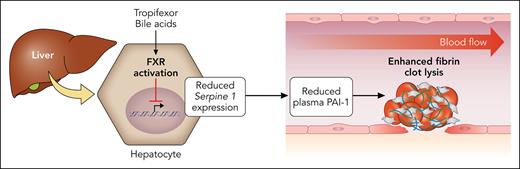
In this issue of Blood, Li et al1 report that tropifexor, an experimental farnesoid X receptor (FXR) agonist, has the potential to improve obesity-associated impairment of fibrinolysis through modulation of hepatic plasminogen activator inhibitor-1 (PAI-1) expression.
Obesity is a complex condition that significantly elevates the risk of developing thrombotic conditions.2 This is due to several factors including inflammation, altered blood flow, endothelial dysfunction and imbalances in hemostasis.3 Obesity is commonly associated with an increase in plasma levels of PAI-1, a key inhibitor of fibrinolysis. PAI-1 interacts with tissue plasminogen activator (tPA) to negatively regulate plasminogen activity. In obesity, both PAI-1 and tPA levels are elevated, but the dominant effect of PAI-1 leads to reduced fibrinolytic activity, increasing the risk of thrombosis.4 These elevations in PAI-1 are driven predominantly by its synthesis in hepatocytes.4,5 Impaired fibrinolysis combined with other prothrombotic factors, contributes to the increased risk of thrombotic and other cardiovascular events in obesity.3 Targeting PAI-1 thus represents a potential therapeutic strategy to reduce thrombotic risk in patients with obesity. However, to date, no inhibitors of PAI-1 have been approved for clinical use.
In this study, the authors demonstrate that hepatocyte-derived PAI-1 expression is negatively regulated by FXR activation. FXR is a nuclear receptor activated by bile acids.6 It is primarily expressed in liver and intestinal cells and acts as a metabolic sensor. Some FXR agonists are already used in the clinic for treatment of other conditions; obeticholic acid is currently used as a second-line treatment for primary biliary cholangitis.7 Synthetic compounds that activate FXR are being explored as potential treatments for other gastrointestinal and metabolic disorders. One such compound is tropifexor, a selective nonbile acid FXR agonist that has already shown promise in clinical trials for treatment of nonalcoholic steatohepatitis.8
The link between FXR and PAI-1 was established using publicly available single-cell transcriptomic data derived from healthy and obese human livers. It was found that Serpine1, the gene encoding PAI-1, was predominantly expressed in hepatocytes. Gene enrichment analysis of differentially expressed genes between the groups revealed “bile acid secretion” as one of the most significantly enriched biological pathways. Moreover, several of the differentially expressed genes were identified as FXR targets. Similar pathway enrichment was observed in other obesity-related transcriptomic data sets from both humans and nonhuman primates.
Diet-induced obesity (DIO) in a mouse model was shown to increase circulating PAI-1 levels and delay fibrinolysis. This model was then employed to assess whether FXR activation could influence hepatic PAI-1 expression. FXR activation using the bile acids chenodeoxycholic acid (CDCA) and taurocholic acid (TCA) lowered PAI-1 expression in primary hepatocytes. These agents also reduced plasma PAI-1 levels and hepatic Serpine1 expression in the DIO mice. In primary hepatocytes derived from FXR-null mice, PAI-1 levels were higher than in wild-type mice, and the absence of FXR prevented the CDCA- or TCA-induced reduction in PAI-1 observed in the wild-type cells. FXR-deficient mice exhibited high circulatory PAI-1 levels, impaired fibrinolysis, and increased deep vein thrombosis (DVT) burden compared to wild-type mice. In Fxrfl/fl mice with AAV8-TBG-Cre, a mouse model where FXR expression is selectively silenced in hepatocytes, elevated plasma PAI-1 levels were also observed relative to controls. Additionally, thrombus length and weight were increased in these animals. Further evidence to support the hypothesis that FXR activation directly represses Serpine1 transcription, was provided by dual luciferase reporter assays and chromatin immunoprecipitation experiments. Moreover, hypofibrinolysis and high DVT load observed in obese mice were improved by tropifexor treatment.
Collectively, this work elegantly demonstrates how hepatic PAI-1 expression may be targeted by FXR activation to reduce thrombotic burden in obesity (see figure). This may represent a long-term therapeutic strategy for reducing DVT risk in individuals with obesity. Although promising, these findings should be interpreted with caution. Murine models are extremely useful for the study of hemostatic pathways. However, although humans and mice share a highly conserved coagulation cascade, there are important differences in how fibrin-clot formation and lysis are regulated between these species. For example, in humans, the fibrin network is denser and more compact,9 whereas mouse plasminogen has lower fibrinolytic efficiency than its human counterpart.10 In this study, the authors also reported that fibrinogen levels remained stable in the DIO mice, whereas slightly elevated fibrinogen levels are typically associated with obesity in humans. This is important as the higher fibrinogen levels in humans with obesity may lead to even denser fibrin networks that are more difficult to break down.
Activation of FXR in hepatocytes by bile acids or tropifexor downregulates expression of Serpine 1, the gene that encodes PAI-1. This results in decreased plasma levels of PAI-1 and enhancement of fibrin-clot lysis. Professional illustration by Patrick Lane, ScEYEnce Studios.
Activation of FXR in hepatocytes by bile acids or tropifexor downregulates expression of Serpine 1, the gene that encodes PAI-1. This results in decreased plasma levels of PAI-1 and enhancement of fibrin-clot lysis. Professional illustration by Patrick Lane, ScEYEnce Studios.
Clinical studies and the use of humanized models will be necessary to assess the therapeutic potential of targeting the FXR/PAI-1 pathway to improve fibrinolysis and reduce DVT risk. Nevertheless, this work highlights an important new mechanism that contributes to hypofibrinolysis in obesity.
Conflict-of-interest disclosure: The author declares no competing financial interests.