In this issue of Blood, Malinverni et al report on outcomes of younger patients with mantle cell lymphoma (MCL) experiencing late relapse, as defined by progression of disease (POD) 24 months after initial diagnosis.1
The overall survival (OS) for patients diagnosed with MCL has improved with currently available therapies, but for most patients, MCL is incurable. There is no standard treatment approach to patients with newly diagnosed MCL, although more intensive treatment with cytarabine-based induction, autologous stem cell transplant (ASCT) consolidation, and rituximab maintenance (RM) achieves prolonged remission durations in patients who tolerate such approaches. Bruton tyrosine kinase inhibitors (BTKis) changed the treatment landscape for patients diagnosed with MCL. For just over a decade, various oral BTKis have been approved in different countries for treatment of relapsed/refractory (r/r) MCL. These agents are active in MCL, with most patients responding to treatment, and have largely become the preferred treatment for patients at initial relapse. However, for younger patients who experience long remissions from intensive treatment approaches, chemoimmunotherapy (CIT) is still used. Malinverni et al evaluated outcomes of younger patients experiencing first, late relapse in this international observational cohort, the LATE-POD study.1
BTKi became a preferred second-line treatment after a pooled analysis of 370 patients with r/r MCL treated with single-agent ibrutinib across 3 separate clinical trials showed improved outcomes when ibrutinib was used as second-line therapy. The median progression-free survival (PFS) was 25.4 months in patients treated with ibrutinib after 1 prior line of therapy (LOT) compared with 10.3 months in patients who had received >1 prior LOT and 12.5 months in the entire cohort of patients.2 Long-term follow-up of this pooled analysis showed an OS of 61.6 months in patients who received ibrutinib as second-line therapy.3 Patients with early POD following frontline CIT achieved a similar median PFS with second-line ibrutinib to that of their initial therapy, and ibrutinib appeared to improve outcomes in early POD compared with CIT based on historical data. Patients with late POD after frontline CIT had an even longer PFS with ibrutinib than with frontline CIT, with a median PFS ≈15 months longer (57.5 months) than the estimated median frontline PFS (42.2 months), but this included only a small group of patients who had received first-line ASCT as consolidation. The MANTLE-FIRST study evaluated outcomes in younger patients treated with high-dose cytarabine-containing regimens and showed that in early POD, patients treated with ibrutinib had significantly better PFS and OS than those treated with CIT; however, in patients with late POD, bendamustine-based regimens achieved similar efficacy as BTKi.4 The benefit of BKTi was also seen in a pooled analysis of 2 studies evaluating the second-generation BTKi zanubrutinib in 112 patients in whom zanubrutinib monotherapy significantly improved OS when used in the second-line vs later-line settings.5
In this article, Malinverni et al report outcomes of 385 patients who received first-line therapy with rituximab and high-dose cytarabine-containing regimens and had late POD treated with either CIT or BTKi.1 For the entire cohort of patients, the median PFS-2 and OS-2 were 33 and 60 months, respectively, at a median follow-up of 53 months from time of first relapse.
The use of BTKi as second-line therapy resulted in a significantly longer median PFS-2 than CIT, with median PFS not reached vs 26 months, respectively (P = .0003). Treatment with BTKi was also associated with significantly better OS-2, with OS not reached with BTKi vs 56 months with CIT (P = .04).
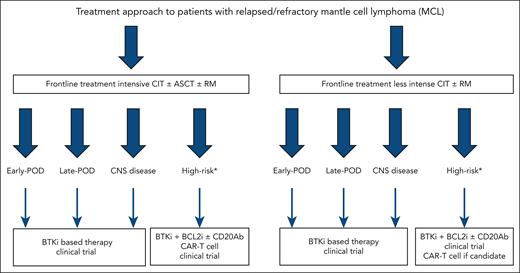
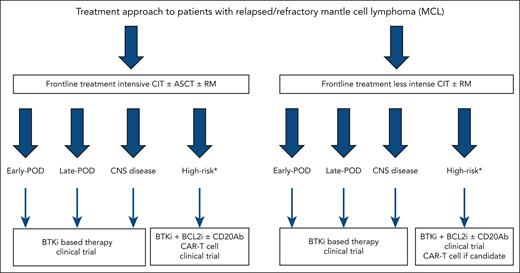
Despite BTKi being the preferred treatment at first relapse for patients with MCL, select patients with prolonged remission durations to initial CIT are treated with alternative CIT regimens. In the LATE-POD study, Malinverni et al show that like patients with early POD after treatment with intensive CIT, those with late POD achieve the best outcomes when treated with BTKi therapy (see figure for proposed treatment algorithm).1 Although R-BAC (rituximab, bendamustine, and low-dose cytarabine) CIT was associated with similar PFS-2 and OS-2 to BTKi, this regimen has historically been associated with higher toxicity. Furthermore, the impact of bendamustine on T-cell function can impact efficacy of chimeric antigen receptor T-cell therapy, approved in the third-line setting, making R-BAC undesirable in this patient population. Despite nearly all patients receiving ibrutinib in this study, there is no reason to believe alternative BTKi would produce inferior results given reported efficacy and improved toxicity profiles of these agents.
Treatment approach to patients with r/r MCL. ∗High-risk refers to MCL with TP53 mutations and/or blastoid or pleomorphic morphology and/or Ki-67 ≥50% (≥30% in select cases). BCL2i, B-cell lymphoma 2 inhibitor; CAR-T, chimeric antigen receptor T-cell therapy; CD20Ab, CD20 monoclonal antibody; CNS, central nervous system.
Treatment approach to patients with r/r MCL. ∗High-risk refers to MCL with TP53 mutations and/or blastoid or pleomorphic morphology and/or Ki-67 ≥50% (≥30% in select cases). BCL2i, B-cell lymphoma 2 inhibitor; CAR-T, chimeric antigen receptor T-cell therapy; CD20Ab, CD20 monoclonal antibody; CNS, central nervous system.
Moving BTKi into frontline treatment has become a priority in MCL, and recent trials have validated incorporation of BTKi into initial therapy improves efficacy. The SHINE trial, a randomized phase 3 trial in older patients evaluating the addition of ibrutinib to CIT with BR (bendamustine and rituximab) and RM, showed ibrutinib significantly improved PFS, although toxicity concerns hindered approval.6 The TRIANGLE study showed the addition of ibrutinib to frontline CIT with or without ASCT improved outcomes with additional toxicity when used after ASCT.7 The LATE-POD study has confirmed that all patients with r/r MCL benefit from treatment with BTKi over CIT. Although frontline trials have shown BTKi improves outcomes when added to CIT, perhaps the best question is if patients with treatment-naïve MCL benefit from the use of BTKi over CIT. Several smaller studies have shown BTKi, in combination with CD20 monoclonal antibodies with or without other targeted therapies, produces high response rates and durable remissions, even achieving high rates of undetectable minimal residual disease. As frontline treatment for MCL evolves, the question should become what is the role of CIT, not what is the role of BTKi-based therapy.
Conflict-of-interest disclosure: K.M. reports consulting fees from AbbVie, ADC Therapeutics, AstraZeneca, Beigene, Bristol Myers Squibb, Genmab, Genentech, Gilead, Incyte, Janssen, Lilly, and MorphoSys.