In this issue of Blood, Naviglio et al show that inflammatory complications of Wiskott-Aldrich syndrome (WAS) are effectively treated with interleukin-1 (IL-1) blockade as a bridge to definitive therapy.1 This is a novel use of agents that are currently approved for arthritis and specific rare monogenic autoinflammatory disorders affecting the inflammasome and arguably the first treatment tailored to the pathobiology of WAS.
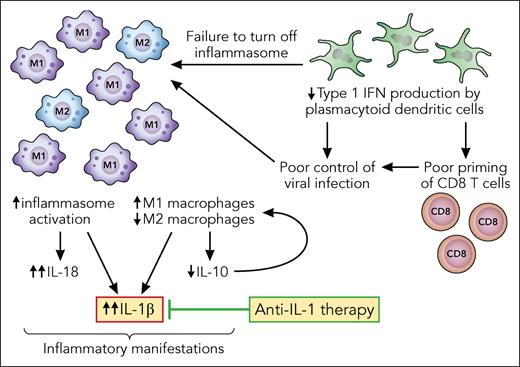
WAS is an X-linked disorder caused by hemizygous mutations in the WAS gene, encoding the WAS protein (WASP). WASP is expressed in hematopoietic cells and is critical for transducing signals that trigger actin cytoskeletal rearrangement, and its complete absence results in clinical manifestations of eczema, microthrombocytopenia, and immunodeficiency. The immunodeficiency spans a spectrum of diseases often lumped together, though the cellular mechanisms differ for specific manifestations. Predisposition to sinopulmonary infections is largely driven by deficiency of T- and B-cell activation downstream of their receptors, leading to poor specific antibody production, particularly to carbohydrate antigens of encapsulated organisms. B-cell intrinsic autoantibody production and deficiency of regulatory T- and B-cell number and function drive autoimmune cytopenias, hepatitis, nephritis, and others. In contrast, defects in myeloid cell function (ie, monocytes, macrophages, and dendritic cells) drive inflammatory disease in WAS, including of the skin (vasculitis panniculitis, pyoderma gangrenosum, and erysipelas), arthritis, and inflammatory bowel disease, sometimes with fever and systemic symptoms reminiscent of autoinflammatory disorders. Several cellular mechanisms result in a final common pathway of IL-1β and IL-18 overproduction in patients with WAS (see figure). Sensing of microbial and other antigens by the sensor NRLP3 in myeloid cells activates the inflammasome, leading to cleavage of procaspase-1 into the active form, which in turn cleaves proproteins to active IL-1β and IL-18. Myeloid cells from mice and humans deficient in WASP have hyperactivation of the inflammasome in response to stimulation, overproduce IL-1β, and fail to phagocytose bacteria, which results in prolonged sterile inflammation.2 Patients with WAS also have higher circulating levels of IL-18.3 Type 1 interferons such as interferon alfa (IFNα), typically produced by dendritic cells in response to viral or other infection, dampen the production of IL-1β and IL-18 by macrophages, promoting resolution of the inflammatory response. In murine models, WASP-deficient plasmacytoid dendritic cells failed to make IFNα in response to cytomegalovirus infection, leading to poor viral control and uncontrolled inflammation.4 We can surmise, therefore, further aberrant inflammasome activity in patients with WAS. Finally, WASP is critical for homeostasis and function of M1 proinflammatory and M2 anti-inflammatory macrophages. M2 macrophages were lower in number in the intestines of Was-deficient mice and aberrantly produced IL-1β and other proinflammatory cytokines while failing to produce the regulatory cytokine IL-10.5 Failure to polarize to the M2 anti-inflammatory phenotype was shown to be cell intrinsic and due to failure of IL-10 signaling.5 Dysregulated overproduction of IL-1β and other proinflammatory cytokines and ensuing effects on endothelial cells, vascular smooth muscle cells, chondrocytes, synovial cells, neutrophils, and other cells result in inflammation, tissue destruction, and clinical symptoms.
WAS-deficient myeloid cells drive excess inflammation. In response to infectious or other stimuli, the inflammasome is activated in macrophages, triggering elaboration of IL-1β, IL-18, and other cytokines. Patients with WAS have an excess of proinflammatory M1 macrophages, and M2 macrophages inappropriately secrete IL-1β. The regulatory cytokine IL-10 requires WASP to signal in macrophages, which results in failure to differentiate into M2 macrophages, an important source of IL-10. Plasmacytoid dendritic cells in patients with WAS are poor producers of type 1 interferon. The lack of type 1 interferons fuels macrophage activation directly by failing to turn off the inflammasome and indirectly by insufficient activation of CD8 T cells needed to clear the original infectious trigger. Professional illustration by Patrick Lane, ScEYEnce Studios.
WAS-deficient myeloid cells drive excess inflammation. In response to infectious or other stimuli, the inflammasome is activated in macrophages, triggering elaboration of IL-1β, IL-18, and other cytokines. Patients with WAS have an excess of proinflammatory M1 macrophages, and M2 macrophages inappropriately secrete IL-1β. The regulatory cytokine IL-10 requires WASP to signal in macrophages, which results in failure to differentiate into M2 macrophages, an important source of IL-10. Plasmacytoid dendritic cells in patients with WAS are poor producers of type 1 interferon. The lack of type 1 interferons fuels macrophage activation directly by failing to turn off the inflammasome and indirectly by insufficient activation of CD8 T cells needed to clear the original infectious trigger. Professional illustration by Patrick Lane, ScEYEnce Studios.
The inflammatory symptoms of WAS are generally poorly responsive to treatment, and autoimmunity (spanning both autoantibody- and innate immunity-related diseases) is a high-risk feature of the disease. The standard definitive treatment for WAS (allogeneic hematopoietic stem cell transplant [HSCT]) and newer treatments (ie, autologous gene therapy [GT]) are both effective at correcting disease manifestations, when high levels of donor or gene-corrected cell engraftment are achieved.6-9 The authors have shown that 7 patients who had partial or no response to steroids had complete or partial response to anti-IL-1 therapy, allowing 3 to proceed to HSCT and 4 to GT. Thus, anti-IL-1 therapy was highly effective for control of inflammatory disease and should be incorporated into treatment of WAS, particularly leading up to definitive therapy.
Given the central role of myeloid cells in the pathogenesis of hyperinflammation in WAS, it stands to reason that correction of these manifestations would require high-level replacement of the myeloid compartment with WASP-expressing cells. After both HSCT and GT, low-level correction in the myeloid compartment has been associated with failure to correct platelet numbers, and after HSCT, autoimmunity post was more frequent in patients with mixed myeloid chimerism.6-10 The threshold of correction needed to protect against autoimmunity is unclear, and previous literature has generally not distinguished the effect of partial myeloid cell correction on autoantibody-mediated vs inflammatory disease. The authors showed an intriguing correlation between myeloid cell correction and protection against recurrent inflammatory disease. Two of the 3 patients who responded to IL-1 blockade and proceeded to HSCT had recurrences of disease in the setting of mixed myeloid donor chimerism. An additional 2 patients first developed inflammatory disease after HSCT, both in the setting of falling myeloid donor chimerism, which was responsive to anti-IL-1 therapy. In 3 patients in whom full donor myeloid chimerism was ultimately achieved, after the initial or after a second HSCT, inflammatory disease resolved without the need for continued IL-1 blockade, and 2 patients with mixed myeloid chimerism remain dependent. Thus, the authors have shown that IL-1 blockade can be effective for management in cases where HSCT has resulted in partial correction of myeloid cells.
The findings in this article represent an important advance in treatment of WAS in preparation for and after definitive therapy. Many questions remain to be answered in future studies. It is interesting that all 4 patients post-GT were free of inflammatory disease with 4 to 8 years of follow-up, even in those with only a fraction of myeloid cells expressing WASP (indicated by vector copy number <1). The reason for this disparate outcome compared with HSCT is unknown and will require more patients undergoing GT to be studied. IL-1 blockade would not be expected to impact excess IL-18 or deficient IL-10 production; combination therapy, including treatment with IFNα to prevent IL-1 elaboration, could be explored in the future.
Conflict-of-interest disclosure: S.-Y.P. declares no competing financial interests.