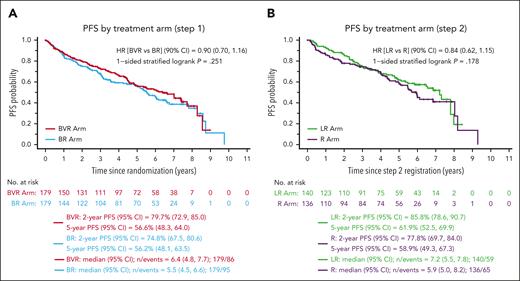
In this issue of Blood, Smith et al show that bendamustine-rituximab (BR) followed by rituximab maintenance (R) represents an effective induction regimen for patients with mantle cell lymphoma (MCL).1 The authors conclude that this combination is viable standard initial therapy, at least for older patients, and can be considered the preferable comparator for developing newer regimens. The present trial had 2 coprimary objectives and 4 arms, aiming at improving progression-free survival (PFS) either with the addition of bortezomib to BR induction or lenalidomide to R maintenance. All 3 experimental arms failed to demonstrate a PFS advantage compared with the backbone BR + R (see figure). Furthermore, bortezomib was reported to increase the risk of neutropenia and sensory neuropathy during induction, and lenalidomide was associated with higher rates of neutropenia, rash, fatigue, and discontinuation of therapy due to adverse events prior to the completion of the planned 24 cycles.
Results from bendamustine-based combinations. Progression-free survival by induction regimen (A) or maintenance regimen (B) in the Smith et al1 randomized trial. B, bendamustine; CI, confidence interval; HR, hazard ratio; LR, lenalidomide, rituximab; V, bortezomib (Velcade); R, rituximab; L, lenalidomide. See Figure 2A-B in the article by Smith et al1 beginning on page 1083.
Results from bendamustine-based combinations. Progression-free survival by induction regimen (A) or maintenance regimen (B) in the Smith et al1 randomized trial. B, bendamustine; CI, confidence interval; HR, hazard ratio; LR, lenalidomide, rituximab; V, bortezomib (Velcade); R, rituximab; L, lenalidomide. See Figure 2A-B in the article by Smith et al1 beginning on page 1083.
The failure of additional therapy in the experimental arms to demonstrate a PFS advantage was potentially due to the excellent performance of the BR + R arm, which was associated with extremely high objective response (88%) and median PFS of 6.9 years. These figures compare favorably with the control arm (BR + R) of the SHINE trial, reporting median PFS of 4.4 years in slightly older patients with similar mantle cell lymphoma international prognostic index (MIPI) risk profile,2 and with the large retrospective cohort of patients treated with BR + R in the US community setting (median PFS 5.4 years),3 where again patients were older than in the present series (median 72.5 vs 68 years). One of the important findings in the latter study was that BR + R was associated with significantly improved overall survival vs BR alone, confirming the conclusions by Smith et al, thus pointing to BR + R as the preferable induction regimen in patients with MCL that are not candidate for more intensive approaches. The double randomized clinical trial by the European Mantle Cell Lymphoma Network reported significantly improved PFS in comparison with R alone for patients in the R + lenalidomide maintenance arm after R-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)-based induction.4 Although there was an increase in adverse events, and no final advantage in overall survival, the PFS advantage observed after R-CHOP indicates that BR, likely due to its immunosuppressive profile, may not be the preferable induction if the goal is to enhance the activity of rituximab-based maintenance therapy. Randomized trials are underway, such as the BGB-311-306 or the ENRICH, that will soon tell us if chemotherapy-free combinations of zanubrutinib or ibrutinib with rituximab, respectively, may overcome the safety and efficacy profile of BR + R.
In the attempt to build on BR, with or without R, several groups have tried to increase the effectiveness of this backbone by adding lenalidomide,5 cytarabine,6 or ibrutinib.2 Notably, the indication for ibrutinib for MCL and marginal zone lymphoma treatment was withdrawn from the market following the SHINE study,2 a phase 3 trial comparing ibrutinib with BR vs BR alone. Despite demonstrating improved PFS, the ibrutinib-BR arm exhibited a higher rate of adverse events and no advantage in overall survival. With all attempts, it seemed that the efficacy of BR was consistently improved, but at the price of a significant increase of toxic events, mainly hematological or infectious complications. A similar phase 3 randomized study, adding acalabrutinib to BR (ACE-LY308), has recently reported preliminary results from an interim analysis, showing a statistically significant improvement in PFS vs BR in previously untreated patients with MCL. Again, we will need to evaluate the potential extended overall survival and if the tolerability of acalabrutinib in combination with BR was consistent with its known safety profile. We expect these data to be presented at forthcoming meetings and shared with global regulatory authorities soon.
The upfront treatment of patients with MCL has typically been based on age, with patients younger than 65 being candidates for intensive regimens followed by consolidation with autologous transplant. This strict dichotomous “age-based” approach, however, has been challenged by the significantly worse outcome in patients with particular histopathological and molecular features, regardless of the therapy used. In the trial by Smith et al, outcome of high-risk patients, defined by Ki67 > 30% or TP53 immunohistochemistry > 50%, was available in only 138 patients, preventing comparison between the 4 arms. Nevertheless, both variables were associated with significantly inferior survival, with disappointing median PFS of approximately 2 years and 1 year, respectively, for the previously defined high-risk populations. The presence of TP53 mutations, among others, has been consistently reported to be associated with inferior outcome after BR or more intensive regimens.2,7 Interestingly, the TRIANGLE study has shown that the addition of ibrutinib to intensive induction immunochemotherapy and R maintenance seems to confer a PFS advantage to high-risk patients as defined in the Smith trial.8 Most recently, the chemotherapy-free combination of zanubrutinib, venetoclax, and obinutuzumab (BOVEN study) in 25 patients of any age with untreated TP53-mutated MCL reported a promising 2-year PFS of 72%.9 Similar efficacy results in patients with TP53 mutations were reported in the V-RBAC trial, where venetoclax was used to consolidate and maintain a bendamustine-based induction (R-BAC).10 It is possible that chemotherapy-free treatments, due to their diverse mechanisms of action, especially when independent of DNA repair pathways, may represent the best choice for these patients, irrespectively of patients’ age.
Thanks to the great work by Smith et al, which reported solid and long-term data on the efficacy of BR + R, we now have an established backbone for the treatment of patients with MCL at standard risk who may not be candidates for intensive regimens.
Conflict-of-interest disclosure: C.V. declares no competing financial interests.