In this issue of Blood, Leopizzi et al have identified 2 novel functions of Epstein-Barr virus (EBV) nuclear antigen 2 (EBNA2),1 which appear to be operative in the context of a subset of EBV-associated B-cell lymphomas. EBV is a fascinating virus, and it is the ultimate viral survivor. How else to characterize a persistent herpes virus that has successfully infected 95% of the world’s population? It achieves this elite status by causing only a relatively modest burden of disease in the vast majority of individuals that it infects. This is accomplished via a highly sophisticated gene apparatus that includes employing multiple, and sometimes contradictory, strategies during its viral life cycle.2 This includes encoding for both proproliferative and antiapoptotic functions, and at different times by being highly immunogenic, and at other times extremely effective at evading the host immune system.
However, in some individuals, EBV does cause serious disease. Indeed, EBV was the first tumor virus identified in humans, and among the cancers most frequently associated with it are B-cell lymphomas. Understanding the function of individual EBV genes that are expressed by EBV-associated B-cell lymphomas can therefore help us discover new therapeutic vulnerabilities for application in precision oncology.
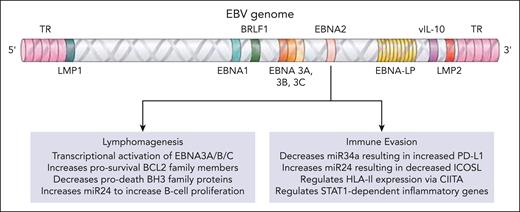
During primary infection, EBV-infected cells are rescued from cell death by EBNA2-driven proproliferative and antiapoptotic gene expression programs. EBNA2 achieves this indirectly, akin to an orchestra conductor that expertly coordinates musicians to produce the perfect balance of the instruments in a symphony, by promoting transcriptional transactivation of viral and cellular genes (see figure). EBNA2 does this by acting as an adapter molecule that binds to cellular sequence-specific DNA-binding proteins. However, the process is normally kept in check, in part because EBNA2 and the viral genes it transactivates (EBNA3A/B/C) are highly immunogenic antigens that are targetable by host EBV-specific CD4+ and CD8+ T cells. Nevertheless, as with the world-renowned maestra in the recent film Tár, things can go horribly awry, and pathology (eg, lymphoma) can result. EBNA2-associated lymphomas include latency III cases of EBV-associated diffuse large B-cell lymphoma not otherwise specified (EBV-associated DLBCL-NOS) that arise in the immunocompetent,3 or as part of monomorphic posttransplant lymphoproliferative disorders (PTLDs) with DLBCL histology.4
EBNA2 is known to have multiple roles that promote lymphomagenesis and enable evasion of the host immune response. BCL2, B-cell lymphoma 2; BH3, Bcl2 homology domain 3; CIITA, class II major histocompatibility complex transactivator; HLA, human leukocyte antigen; ICOSL, inducible T-cell costimulator ligand; LMP1, latent membrane protein 1; PD-L1, programmed death ligand 1; TR, terminal repeat; vIL-10, viral interleukin 10.
EBNA2 is known to have multiple roles that promote lymphomagenesis and enable evasion of the host immune response. BCL2, B-cell lymphoma 2; BH3, Bcl2 homology domain 3; CIITA, class II major histocompatibility complex transactivator; HLA, human leukocyte antigen; ICOSL, inducible T-cell costimulator ligand; LMP1, latent membrane protein 1; PD-L1, programmed death ligand 1; TR, terminal repeat; vIL-10, viral interleukin 10.
The team from Sapienza University in Rome has previously shown that EBNA2 upregulates the immune checkpoint ligand programmed death ligand 1 (PD-L1) by downregulating the microRNA miR-34a in B-cell lymphomas.5 Using a series of elegant approaches, including EBNA2-expressing and EBNA2-nonexpressing B-cell lines, they now show that EBNA2 increases expression of miR-24.1 The impacts of this are twofold. First, they show that the 3’ untranslated region of inducible T-cell costimulator ligand (ICOSL) is a validated target of miR-24. Hence, EBNA2 can indirectly reduce expression of this ligand to ICOS (CD278), an activating costimulatory immune checkpoint expressed on activated T cells, regulatory T cells, and T-follicular helper cell populations. Not only does this add to the relatively spartan data so far regarding the ICOS/ICOSL pathway in the context of B-cell lymphomas, but it also provides a potential highly targeted microRNA-mediated approach to switching off immune checkpoints. Here, a combined line of attack could be employed using reconstitution of miR-34a with mimics and downregulation of miR-24 by anti–miR-24 molecules. This would overcome immune evasion caused by high PD-L1 and low ICOSL expression in EBNA2-associated B-cell lymphomas. This strategy could be beneficial in EBV-associated DLBCL-NOS, an aggressive B-cell lymphoma that is known to have a highly tolerogenic tumor microenvironment.3 However, unless such an approach was limited to EBNA2-expressing cells, it would likely be clinically contraindicated in EBNA2-associated PTLD as reversal of immune tolerance would increase the risk of organ transplant rejection.
Second, Leopizzi et al show that miR-24 is responsible for the fine-tuning required to maintain proproliferative levels of c-MYC.1 The balance between c-MYC–driven proliferation and apoptosis is tipped in favor of proliferation in the setting of MYC-driven cancers. In the context of c-MYC–driven B lineage tumors, this delicate balance has been readily demonstrated using the Eμ-Myc transgenic mouse model of B-cell leukemia/lymphoma,6 where increased expression of antiapoptotic B-cell lymphoma 2 (BCL-2) family proteins accelerates c-MYC–driven lymphoma onset,7 and reduced expression of antiapoptotic BCL-2 family proteins leads to apoptosis and lymphoma regression.8 Although miR-24 has been reported to regulate c-MYC,9 here Leopizzi et al show that EBNA2 induces miR-24 to maintain c-MYC at levels that drive proliferation but remain below the threshold needed to induce apoptosis.1 The team further show that, in a nuts and bolts approach, levels of antiapoptotic BCL-2 are maintained by miR-24.1 Whether miR-24 elicits broader effects on the apoptosis machinery in this context, including regulation of proapoptotic members, as has been reported for BCL2L11 (BIM),10 remains to be determined. This important study by Leopizzi et al opens up new avenues for future research, assuming the results are validated in large cohorts of primary samples of EBV-associated B-cell lymphomas. The mechanisms by which EBNA2 upregulates miR-24 are an open question. Clinically, the observation that EBV, through the EBNA2/miR24 axis, can regulate BCL-2 levels may indicate that these EBV-associated lymphomas could have an increased dependency on BCL-2 and, as such, Bcl2 homology domain 3 (BH3)-mimetic drugs, like venetoclax, which targets BCL-2, may impart therapeutic benefit. From the perspective of immune therapy, the use of emerging EBV-infected humanized mouse models would also be important to test the potential efficacy of miR-34a mimic/anti–miR-24–based therapeutics.
Conflict-of-interest disclosure: M.K.G. declares no competing financial interests. G.L.K. is an employee of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax.