Key Points
Hepatocyte-specific Notch1 deficiency impairs hepatic TPO production and reduces circulating platelet number.
Delta-like 4 on desialylated platelets activates hepatocyte Notch1 signaling and upregulates HES5, leading to JAK2/STAT3 phosphorylation.
Visual Abstract
Notch signaling regulates cell-fate decisions in several developmental processes and cell functions. However, the role of Notch in hepatic thrombopoietin (TPO) production remains unclear. We noted thrombocytopenia in mice with hepatic Notch1 deficiency and so investigated TPO production and other features of platelets in these mice. We found that the liver ultrastructure and hepatocyte function were comparable between control and Notch1-deficient mice. However, the Notch1-deficient mice had significantly lower plasma TPO and hepatic TPO messenger RNA levels, concomitant with lower numbers of platelets and impaired megakaryocyte differentiation and maturation, which were rescued by addition of exogenous TPO. Additionally, JAK2/STAT3 phosphorylation was significantly inhibited in Notch1-deficient hepatocytes, consistent with the RNA-sequencing analysis. JAK2/STAT3 phosphorylation and TPO production was also impaired in cultured Notch1-deficient hepatocytes after treatment with desialylated platelets. Consistently, hepatocyte-specific Notch1 deletion inhibited JAK2/STAT3 phosphorylation and hepatic TPO production induced by administration of desialylated platelets in vivo. Interestingly, Notch1 deficiency downregulated the expression of HES5 but not HES1. Moreover, desialylated platelets promoted the binding of HES5 to JAK2/STAT3, leading to JAK2/STAT3 phosphorylation and pathway activation in hepatocytes. Hepatocyte Ashwell-Morell receptor (AMR), a heterodimer of asialoglycoprotein receptor 1 [ASGR1] and ASGR2, physically associates with Notch1, and inhibition of AMR impaired Notch1 signaling activation and hepatic TPO production. Furthermore, blockage of Delta-like 4 on desialylated platelets inhibited hepatocyte Notch1 activation and HES5 expression, JAK2/STAT3 phosphorylation, and subsequent TPO production. In conclusion, our study identifies a novel regulatory role of Notch1 in hepatic TPO production, indicating that it might be a target for modulating TPO level.
Introduction
Thrombopoietin (TPO) regulates megakaryocyte development and maturation and platelet production, through binding to its receptor myeloproliferative leukemia virus oncogene.1 Although TPO can be secreted from the spleen, kidney, or stromal cells in the bone marrow (BM),2 it is predominantly produced by the liver.3 However, there are few studies on the molecular mechanism by which TPO is generated and regulated under physiological conditions. TPO level can be removed from the circulation after binding to TPO receptors, leading to internalization and subsequent destruction.4,5 In addition, the binding of aged and desialylated platelets to Ashwell-Morell receptors (AMR) on hepatocytes can stimulate hepatic TPO production.6 A second study revealed that the extracellular domain of platelet glycoprotein Ibα (GPIbα) was required for hepatic TPO generation.7
The Notch signaling pathway is highly conserved and plays critical roles in cell-fate decisions in numerous developmental processes and cell functions.8 In mammals, 4 Notch receptors (Notch1-4) and 5 ligands (Delta-like 1, Delta-like 3, Delta-like 4 [Dll4], and Jagged-1/2) have been identified.9 Notch signaling regulates physically adjacent cell-cell interactions through engagement of Notch receptors by their cognate ligands, which induces proteolytic cleavage of Notch, leading to the release of the Notch intracellular domain, which is then translocated to the nucleus where it regulates gene transcription and expression.10,11 In the hematopoietic system, Notch positively regulates megakaryopoiesis and cell-fate decisions among myeloid progenitors.12 However, Notch/Delta4 signaling has been shown to inhibit human megakaryocytic terminal differentiation.13 Whether Notch governs hepatic TPO production remains unknown. In this study, hepatocyte-specific Notch1 knockout mice were used to characterize the role of Notch1 in the production of hepatic TPO.
Methods
Animals
Notch1flox/flox mice (Notch1fl/fl) were purchased from The Jackson Laboratory (strain number 007181). The hepatocyte-specific Notch1 knockout mice (hepatocyte [HC] Notch1−/−) were generated by crossing Notch1flox/flox mice with transgenic mice expressing albumin promoter-driven Cre recombinase (The Jackson Laboratory; strain number 003574). Notch1 deletion in hepatocyte was verified by western blot (supplemental Figure 1, available on the Blood website). This study was approved by the Animal Ethics Committee of Xuzhou Medical University. Other methods are described in the supplemental Data.
TPO level detection
Plasma TPO level was measured by using a enzyme-linked immunosorbent assay kit (mouse TPO, TPO enzyme-linked immunosorbent assay kit; Cusabio). Liver TPO messenger RNA (mRNA) level was detected by quantitative real-time polymerase chain reaction. The primer for TPO was as follows: forward, 5’-TGGGGAAGGGATACACAGCA-3’, and reverse, 5’-GACCAAAGATCGCTAGCTGCTC-3’.
Platelet half-life measurement
Mouse platelet half-life or clearance in vivo was evaluated through the administration of Dylight 488–labeled anti-GPIbβ antibody (Emfret, X488) via tail vein (0.1 μg/g body weight). The proportion of fluorescently labeled platelets after administration was monitored by flow cytometry as described previously.14
Exogenous TPO administration
Mice received an intraperitoneal administration of exogenous TPO (Stemcell Technologies; 1 μg/kg) consecutively for 5 days, followed by relevant analysis.
Preparation of desialylated platelets
Mouse platelets (1.2 × 108/mL) were treated with neuraminidase from Clostridium perfringens (2.5 mU; Roche) for 15 minutes at 37°C to remove terminal sialic acid. Platelet desialylation was verified by flow cytometry using fluorescein isothiocyanate–labeled Ricinus communis (castor bean) agglutinin I (RCA I; Invitrogen).
Primary hepatocytes isolation and treatment
After anesthesia, a catheter was inserted into inferior vena cava, and liver was perfused first with divalent cation-free Dulbecco modified Eagle medium buffer with EGTA for 5 to 10 minutes at 37°C, and then with collagenase type Ⅳ (Gibco) for 3 to 5 minutes. The liver was excised and dissociated in Dulbecco's modified Eagle medium supplemented with 4% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 15 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.1 μM dexamethasone, and 4 μg/mL insulin. After that, hepatocytes were filtered through a 70-μm nylon mesh, washed, purified by Percoll (Solarbio, Beijing, China) gradient centrifugation, and transferred to a 60 mm × 15 mm style culture dish coating Rat Tail Tendon Collagen Type Ⅰ (Solarbio) at 37°C for 4 hours. The adherent hepatocytes were washed gently using phosphate-buffered saline and cultured with desialylated platelets (1:10) for 30 minutes or 60 minutes followed by washing and centrifugation to collect cell lysates. For some experiments, desialylated platelets were pretreated with anti-Dll4 antibody (1 μg/mL; ThermoFisher Scientific) for 5 minutes and then incubated with hepatocytes. In addition, the isolated primary hepatocytes were treated with asialofetuin (200 μg/mL; Sigma-Aldrich) for 15 minutes to block AMR or anti-asialoglycoprotein receptor1 (ASGR1) antibody (5 μg/mL; Proteintech) for 5 minutes to block ASGR1, followed by incubation with desialylated platelets.
Statistical analysis
Data are presented as mean ± standard deviation or standard error where indicated and analyzed by student t test or 1- or 2-way analysis of variance. P value <.05 indicates a significant difference.
This study was approved by the Animal Ethics Committee of Xuzhou Medical University.
Results
Hepatocyte Notch1 deficiency causes thrombocytopenia and reduces TPO level
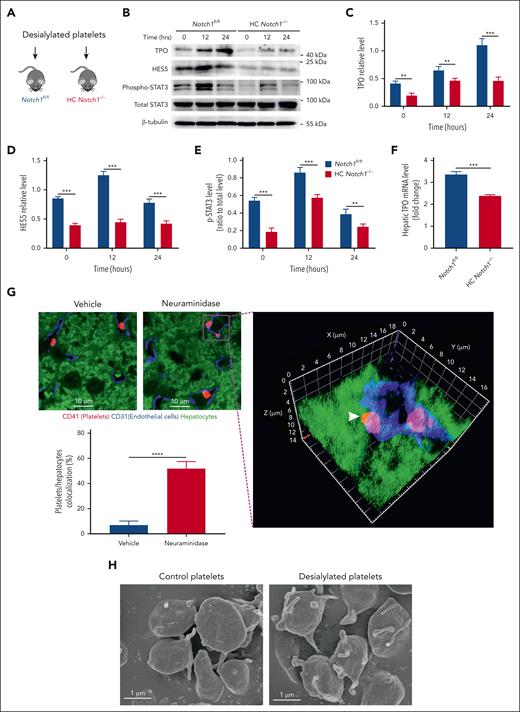
Considering the role of Notch1 signaling in cell-fate decisions, we first evaluated whether Notch1 deficiency in hepatocytes affects liver ultrastructure and function. Liver sections were isolated to assess the histological changes of liver tissues by hematoxylin and eosin staining, and the results showed no obvious abnormalities in liver sections bearing hepatocyte Notch1 deficiency (supplemental Figure 2A). Staining showed hepatocytes uniformly arranged radially around the central vein, forming a hepatic cord, with presence of normal hepatic sinusoids (supplemental Figure 2A). Consistently, hepatocyte Notch1 deficiency did not alter liver function as demonstrated by normal levels of aspartate transaminase (supplemental Figure 2B), alanine aminotransferase (supplemental Figure 2C), total bilirubin (supplemental Figure 2D), albumin (supplemental Figure 2E), globulin (supplemental Figure 2F), albumin/globulin (supplemental Figure 2G), direct bilirubin (supplemental Figure 2H), and total protein (supplemental Figure 2I) between Notch1fl/fl mice and HC Notch1−/− mice, consistent with a previous study demonstrating that hepatocyte deletion of Notch1 did not alter liver architecture and bile duct structures.15 However, the number of peripheral circulating platelets (Figure 1A) and white blood cells (supplemental Figure 3A) was significantly reduced in HC Notch1−/− mice compared with control mice. Surprisingly, no abnormalities of red blood cell number (supplemental Figure 3B), plasma level of erythropoietin (supplemental Figure 3C) or mean platelet volume (MPV; supplemental Figure 3D), platelet distribution width (supplemental Figure 3E), and plateletcrit (supplemental Figure 3F) were detected. In addition, BM hematopoietic cell analysis showed significantly reduced numbers of Lin– Sca-1+ c-Kit+ cells, short-term hematopoietic stem cells, common myeloid progenitors, granulocyte/monocyte progenitors, and megakaryocyte progenitors in HC Notch1−/− mice compared with those in control mice, without differences in multipotential progenitor, long-term hematopoietic stem cells, and megakaryocyte/erythrocyte progenitors (supplemental Figure 4). To evaluate whether enhanced platelet clearance contributed to the observed thrombocytopenia in HC Notch1−/− mice, we measured the lifespan of antiglycoprotein Ib (GPIb)–labeled platelets and found normal platelet clearance in HC Notch1−/− mice (supplemental Figure 5). In addition, no increased platelet activation (supplemental Figure 6) or platelet-neutrophil complex formation (supplemental Figure 7) was observed in HC Notch1−/− mice compared with Notch1fl/fl mice. Consistently, platelet functional analysis in vitro also revealed no significant differences in platelet aggregation (supplemental Figure 8A-B) and clot retraction (supplemental Figure 8C) between HC Notch1−/− mice and Notch1fl/fl mice. Interestingly, the rebound thrombopoiesis was significantly ablated in a model of acute thrombocytopenia induced by intraperitoneal administration of antiplatelet antibody (Figure 1B) in HC Notch1−/− mice. Consistent with thrombocytopenia, HC Notch1−/− mice presented a significantly lower plasma level (Figure 1C) and hepatic mRNA level (Figure 1D) of TPO than Notch1fl/fl mice. These results demonstrated that hepatocyte-specific Notch1 deletion reduces the number of circulating platelets and hepatic TPO production without affecting liver function.
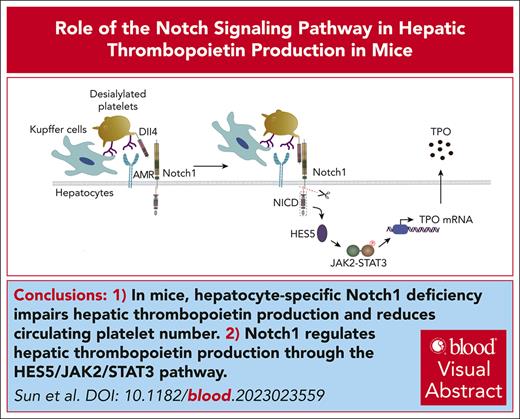
Notch1 deficiency reduces the number of circulating platelet and TPO production. (A) Peripheral blood was drawn to measure the number of circulating platelets by an automatic blood analyzer (mean; n = 17; unpaired student t test). (B) Notch1fl/fl or HC Notch1−/− mice received intraperitoneal administration of rat anti-mouse integrin GPIIb/CD41 immunoglobulin at a dose of 0.1 mg/kg, followed by monitoring the number of circulating platelets at different time points (mean ± standard error of the mean [SE]; n = 7; 2-way analysis of variance [ANOVA]). Plasma (C) or liver mRNA (D) was isolated from Notch1fl/fl or HC Notch1−/− mice to measure TPO level by ELISA (mean ± SE; n = 3) or quantitative real-time PCR (mean ± SE; n = 8; unpaired student t test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction.
Notch1 deficiency reduces the number of circulating platelet and TPO production. (A) Peripheral blood was drawn to measure the number of circulating platelets by an automatic blood analyzer (mean; n = 17; unpaired student t test). (B) Notch1fl/fl or HC Notch1−/− mice received intraperitoneal administration of rat anti-mouse integrin GPIIb/CD41 immunoglobulin at a dose of 0.1 mg/kg, followed by monitoring the number of circulating platelets at different time points (mean ± standard error of the mean [SE]; n = 7; 2-way analysis of variance [ANOVA]). Plasma (C) or liver mRNA (D) was isolated from Notch1fl/fl or HC Notch1−/− mice to measure TPO level by ELISA (mean ± SE; n = 3) or quantitative real-time PCR (mean ± SE; n = 8; unpaired student t test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction.
Impaired megakaryocytes maturation in hepatocyte Notch1-deficient mice
Given the reduced TPO production and thrombocytopenia in HC Notch1−/− mice, we evaluated the maturation of megakaryocytes. Consistently, we found that the BM megakaryocytes presented impaired maturation (Figure 2A) as demonstrated by the reduced number of mature megakaryocytes (Figure 2B) and proportion of high ploidy (≥32N) megakaryocytes (supplemental Figure 9) and the increased number of immature megakaryocytes (Figure 2C) and proportion of low ploidy (≤8N) megakaryocytes (supplemental Figure 9). This might be due to the reduced TPO level because TPO plays a critical role in the regulation of megakaryocytes. To further evaluate whether there is indeed a dysfunction in the megakaryocytes, CD117+ hematopoietic stem cells were isolated from Notch1fl/fl or HC Notch1−/− mice, and their differentiation into megakaryocytes was induced in vitro in the culture medium containing TPO. Surprisingly, we found normal maturation and differentiation of the megakaryocytes from CD117+ hematopoietic stem cells of HC Notch1−/− mice as shown by the megakaryocyte ploidy formation (supplemental Figure 10A) and platelet production (supplemental Figure 10B), indicating that the abnormal maturation of megakaryocytes in HC Notch1−/− mice was due to the reduced TPO levels rather than the dysfunction of megakaryocytes themselves. Further supporting this contention, exogenous supplementation of TPO promoted the maturation of megakaryocytes in HC Notch1−/− mice (Figure 2A; supplemental Figure 9) and significantly increased the number of mature megakaryocytes (Figure 2B) and circulating platelets (Figure 2D).
Impaired megakaryocyte maturation in hepatocyte Notch1-deficient mice. Femurs were isolated from Notch1fl/fl mice or HC Notch1−/− mice treated with vehicle or exogenous TPO (1 μg/kg) for consecutive 5 days and then fixed followed by H&E staining to evaluate megakaryocyte maturation and differentiation (A). Blue arrows indicate the mature megakaryocytes (defined as megakaryocytes having 4-16 nuclear lobes), and red arrows indicate the immature megakaryocytes (defined as megakaryocytes lacking lobulation of the nucleus or <4 nuclear lobes). The number of mature (B) or immature (C) megakaryocytes was quantified (mean ± SE; n = 6; 1-way ANOVA). (D) The number of circulating platelets was measured after supplementation of exogenous TPO (1 μg/kg) for consecutive 5 days (paired student t test). ∗∗∗P < .001.
Impaired megakaryocyte maturation in hepatocyte Notch1-deficient mice. Femurs were isolated from Notch1fl/fl mice or HC Notch1−/− mice treated with vehicle or exogenous TPO (1 μg/kg) for consecutive 5 days and then fixed followed by H&E staining to evaluate megakaryocyte maturation and differentiation (A). Blue arrows indicate the mature megakaryocytes (defined as megakaryocytes having 4-16 nuclear lobes), and red arrows indicate the immature megakaryocytes (defined as megakaryocytes lacking lobulation of the nucleus or <4 nuclear lobes). The number of mature (B) or immature (C) megakaryocytes was quantified (mean ± SE; n = 6; 1-way ANOVA). (D) The number of circulating platelets was measured after supplementation of exogenous TPO (1 μg/kg) for consecutive 5 days (paired student t test). ∗∗∗P < .001.
Reduced phosphorylation of JAK2 and STAT3 in Notch1-deficient hepatocytes
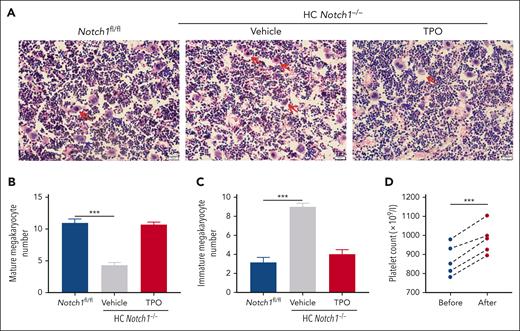
To further investigate the molecular mechanism by which Notch1 modulates hepatic TPO production, we isolated liver from Notch1fl/fl or HC Notch1−/− mice and performed RNA-sequencing analysis and found 266 upregulated genes and 250 downregulated genes (Figure 3A; supplemental Dataset 1). KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway–based analysis of these dysregulated genes identified that JAK/STAT signaling was 1 of several enriched signaling pathways (Figure 3B). This implied that JAK/STAT signaling might be impaired in hepatocyte-specific Notch1-deficient mice, consistent with the role of JAK/STAT signaling in the regulation of TPO production.6 In accordance with RNA-sequencing data, JAK/STAT phospho-signaling was significantly inhibited in the Notch1−/− hepatocytes compared with control hepatocytes, as demonstrated by the reduced phosphorylation of JAK2 and STAT3 (Figure 3C). In addition, we also found a significantly reduced TPO protein expression in the Notch1−/− hepatocytes (Figure 3C), consistent with the lower TPO plasma level (Figure 1C) and hepatic mRNA level (Figure 1D).
Reduced JAK2-STAT3 phosphorylation in Notch1-deficient hepatocytes. (A) The total RNA was isolated from liver of Notch1fl/fl mice or HC Notch1−/− mice and subjected into RNA-sequencing analysis. Differential gene expression between Notch1fl/fl and HC Notch1−/− group was analyzed and presented as volcano map. The x-axis shows the fold change, and the y-axis shows the P value. Red dots indicated the differentially upregulated genes with significance, and green dots presented the differentially downregulated genes with significance. (B) Signaling pathways enriched from KEGG pathway–based analysis. (C) Hepatocyte was isolated from Notch1fl/fl and HC Notch1−/− mice to measure the phosphorylation of JAK2/STAT3 and TPO level by western blot (mean ± standard deviation [SD]; n = 3; unpaired student t test). (D) Cultured hepatocytes were incubated with desialylated platelets for 30 minutes or 60 minutes, followed by measuring the phosphorylation of JAK2/STAT3, TPO level, Notch1 activation (cleaved Notch1 expression), and HES5 expression by western blot (mean ± SD; n = 3; 2-way ANOVA). (E) Expression of HES1 and HES5 in the hepatocyte of Notch1fl/fl and HC Notch1−/− mice was detected by western blot (mean ± SD; n = 3; unpaired student t test). The phosphorylation level of JAK2 and STAT3 was quantified as a ratio relative to respective total level, and the expression of TPO, HES5, HES1, and cleaved Notch1 was quantified as a ratio relative to β-actin level. ∗∗P < .01; ∗∗∗P < .001. ABC, ATP-binding cassette; PPAR, peroxisome proliferator-activated receptor; TGF-β, transforming growth factor-β.
Reduced JAK2-STAT3 phosphorylation in Notch1-deficient hepatocytes. (A) The total RNA was isolated from liver of Notch1fl/fl mice or HC Notch1−/− mice and subjected into RNA-sequencing analysis. Differential gene expression between Notch1fl/fl and HC Notch1−/− group was analyzed and presented as volcano map. The x-axis shows the fold change, and the y-axis shows the P value. Red dots indicated the differentially upregulated genes with significance, and green dots presented the differentially downregulated genes with significance. (B) Signaling pathways enriched from KEGG pathway–based analysis. (C) Hepatocyte was isolated from Notch1fl/fl and HC Notch1−/− mice to measure the phosphorylation of JAK2/STAT3 and TPO level by western blot (mean ± standard deviation [SD]; n = 3; unpaired student t test). (D) Cultured hepatocytes were incubated with desialylated platelets for 30 minutes or 60 minutes, followed by measuring the phosphorylation of JAK2/STAT3, TPO level, Notch1 activation (cleaved Notch1 expression), and HES5 expression by western blot (mean ± SD; n = 3; 2-way ANOVA). (E) Expression of HES1 and HES5 in the hepatocyte of Notch1fl/fl and HC Notch1−/− mice was detected by western blot (mean ± SD; n = 3; unpaired student t test). The phosphorylation level of JAK2 and STAT3 was quantified as a ratio relative to respective total level, and the expression of TPO, HES5, HES1, and cleaved Notch1 was quantified as a ratio relative to β-actin level. ∗∗P < .01; ∗∗∗P < .001. ABC, ATP-binding cassette; PPAR, peroxisome proliferator-activated receptor; TGF-β, transforming growth factor-β.
Platelet desialylation is reported to trigger hepatic TPO transcription and translation through the engagement of hepatic AMR.6 To assess whether this pathway was affected by Notch1 deficiency, hepatocytes were isolated from Notch1fl/fl or HC Notch1−/− liver, and the contamination of Kupffer cells in the isolated hepatocytes was validated as shown by an absence of the expression of mouse Kupffer cell markers F4/80 and CD11b (supplemental Figure 11), indicating no contamination of Kupffer cells in the isolated hepatocytes. The isolated hepatocytes were treated with the desialylated murine platelets, and the results showed that Notch1fl/fl hepatocytes treated with desialylated platelets resulted in an increase of JAK2/STAT3 phosphorylation and TPO production, which were all inhibited in Notch1-deficient hepatocytes (Figure 3D), further confirming the role of Notch1 in the modulation of hepatic TPO production. In addition, we also monitored the expression of HES1 and HES5, 2 intracellular effectors of Notch signaling, and found a significantly reduced expression of HES5 but not HES1 in Notch1-deficient hepatocytes (Figure 3E), implying that HES5 might be involved in the hepatic TPO production. Consistently, HES5 expression was significantly increased in Notch1fl/fl hepatocytes treated with desialylated platelets along with an elevation of TPO production and Notch1 activation (as demonstrated by the increased expression of cleaved Notch1) but not in Notch1-deficient hepatocytes (Figure 3D). These data show that Notch1/HES5 modulates hepatic TPO production possibly through JAK2/STAT3 signaling.
Hepatocyte Notch1 deletion impairs hepatic TPO production in vivo
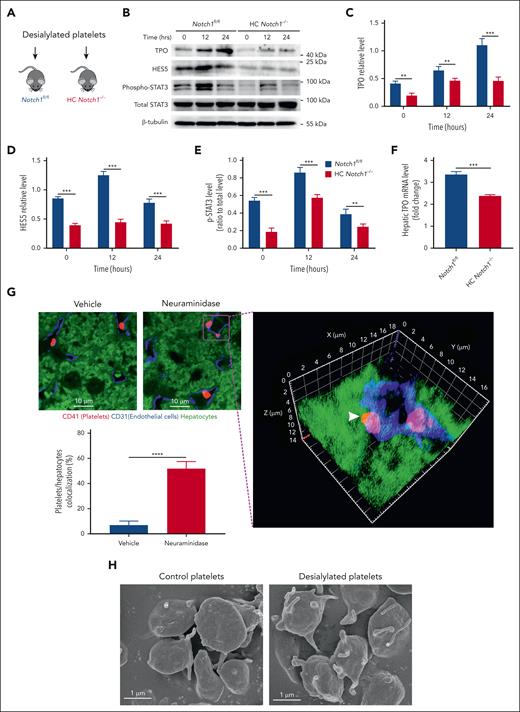
Above results indicate the role of Notch1 signaling in the hepatic TPO production in cell culture system in vitro. To confirm the dependence of hepatic TPO mRNA expression in situ on Notch1, desialylated platelets were transfused into Notch1fl/fl or HC Notch1−/− mice (Figure 4A). To exclude the possible effect of desialylation on platelet function, we measured platelet aggregation and thrombin-mediated clot retraction and found unaltered function of desialylated platelets compared with that of vehicle-treated platelets (supplemental Figure 12). As seen in Figure 4B, after administration of desialylated platelets, a significantly higher hepatic TPO protein expression (Figure 4C) was found in Notch1fl/fl mice than that in HC Notch1−/− mice, along with an increase of HES5 expression (Figure 4D) and STAT3 phosphorylation (Figure 4E). Consistent with the higher hepatic TPO protein expression, Notch1fl/fl mice also presented significantly higher TPO mRNA levels than HC Notch1−/− mice after treatment with desialylated platelets (Figure 4F). These results further support the role of hepatocyte Notch1 in the regulation of in vivo hepatic TPO production.
Hepatocyte Notch1 deletion impairs hepatic TPO production in vivo. Normal platelets were desialylated, and a total number of 2.5 × 108 desialylated platelets was infused into Notch1fl/fl and HC Notch1−/− mice (A), followed by isolation of the liver protein (B) to measure the expression of TPO (C), HES5 (D), and phosphorylation of STAT3 (E) by western blot (mean ± SD; n = 3; 2-way ANOVA) or extraction of liver RNA to detect the TPO mRNA level 24 hours after infusion of desialylated platelets (fold change relative to the basal level) by quantitative PCR assay (mean ± SD; n = 3; unpaired student t test) (F). (G) Wild-type mice were IV injected with vehicle or neuraminidase (50 mU/mouse) to induce in vivo desialylation. After 2 hours, liver was isolated for immunostaining of platelets (CD41, red), liver sinusoidal endothelial cells (CD31, blue), or hepatocytes (autofluorescence, green). The images were analyzed by confocal microscopy (100× oil objective). To observe the colocalization of platelets with hepatocytes, 100× 3-dimensional z stacked image was reconstructed. Arrowhead indicates the colocalization of a platelet with hepatocytes. Platelets/hepatocytes colocalization (%) was quantified as the number of colocalized platelets relative to the total number of platelets in each observed field (100×) (mean ± SE; n = 5-6; unpaired student t test). (H) Untreated or desialylated platelets were fixed, dehydrated, dried, and coated with gold, followed by analysis of the structural changes under a scanning electron microscopy (20 000×) (Representative images were shown from 3 independent experiments). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Hepatocyte Notch1 deletion impairs hepatic TPO production in vivo. Normal platelets were desialylated, and a total number of 2.5 × 108 desialylated platelets was infused into Notch1fl/fl and HC Notch1−/− mice (A), followed by isolation of the liver protein (B) to measure the expression of TPO (C), HES5 (D), and phosphorylation of STAT3 (E) by western blot (mean ± SD; n = 3; 2-way ANOVA) or extraction of liver RNA to detect the TPO mRNA level 24 hours after infusion of desialylated platelets (fold change relative to the basal level) by quantitative PCR assay (mean ± SD; n = 3; unpaired student t test) (F). (G) Wild-type mice were IV injected with vehicle or neuraminidase (50 mU/mouse) to induce in vivo desialylation. After 2 hours, liver was isolated for immunostaining of platelets (CD41, red), liver sinusoidal endothelial cells (CD31, blue), or hepatocytes (autofluorescence, green). The images were analyzed by confocal microscopy (100× oil objective). To observe the colocalization of platelets with hepatocytes, 100× 3-dimensional z stacked image was reconstructed. Arrowhead indicates the colocalization of a platelet with hepatocytes. Platelets/hepatocytes colocalization (%) was quantified as the number of colocalized platelets relative to the total number of platelets in each observed field (100×) (mean ± SE; n = 5-6; unpaired student t test). (H) Untreated or desialylated platelets were fixed, dehydrated, dried, and coated with gold, followed by analysis of the structural changes under a scanning electron microscopy (20 000×) (Representative images were shown from 3 independent experiments). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Although direct in vitro incubation of desialylated platelets with hepatocytes can stimulate TPO production, it is unclear how this might occur, because the circulating platelets in the liver sinusoids are separated from hepatocytes by a fenestrated endothelial cell barrier (∼180 nm in size),16 which makes it impossible for murine circulating platelets (500 nm in diameter) to cross the endothelial barrier to directly interact with hepatocytes in vivo.17 However, a previous study showed the presence of hepatocyte microvilli in the liver sinusoid lumen through sinusoid endothelial fenestrae, providing a possibility for the interaction of hepatocytes with platelets.18 Instead of hepatocytes, liver Kupffer cells, residing on the luminal side of the sinusoids, are shown to directly bind to desialylated platelets and be responsible for the clearance of desialylated platelets.19-21 To evaluate whether there is a direct interaction between desialylated platelets and hepatocytes, we performed confocal microscopic analysis of the liver sections and found that platelets are localized within the liver sinusoids and could not come across the fenestrations to come in contact with hepatocytes (Figure 4G). However, a colocalization of desialylated platelets with hepatocytes (arrowhead, Figure 4G) was observed at the interface between hepatocytes and liver sinusoidal endothelial cells in the liver sections from mice treated with neuraminidase, which induces in vivo desialylation. Interestingly, the majority of a platelet is attached to the liver sinusoidal endothelial cells, implying that the plasma membrane of desialylated platelets might have extensions that can come across endothelial fenestrations to directly interact with hepatocytes. Of note, effector CD8+ T cells can probe the subsinusoidal hepatocytes for the presence of antigens by extending cytoplasmic protrusions through endothelial fenestrae during hepatotropic viral infections.22 To verify this, we compared the morphological changes induced by desialylation of platelets by using scanning electron microscopy and found extended protrusions on the surface of desialylated platelets (Figure 4H), supporting the hypothesis that desialylated platelets have a chance to directly interact with hepatocytes through the extended protrusions. Further studies are required to confirm that the protrusions of desialylated platelets can come across the endothelial fenestrae to interact with the hepatocyte in vivo by using a previously reported correlative technique that combines the transmission electron tomography with the 3-dimensional confocal fluorescence microscopy.22
HES5 binds to JAK2/STAT3 and promotes their phosphorylation
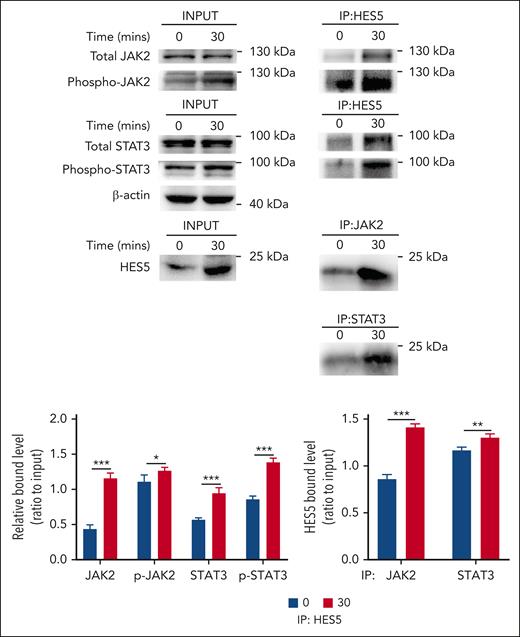
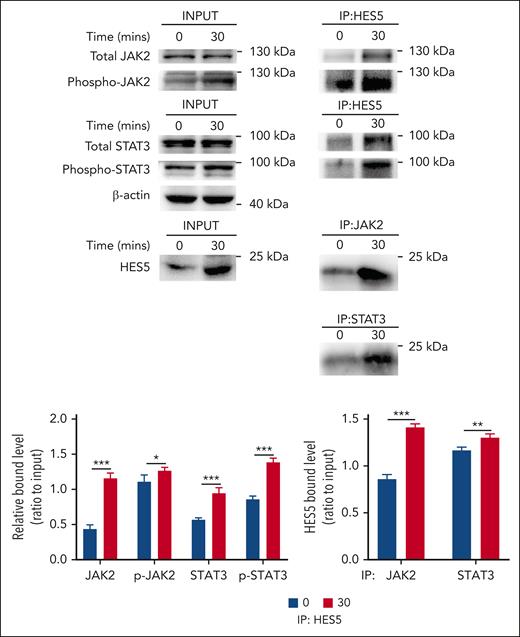
A previous study demonstrated that the binding of HES to STAT3 modulates the cross talk between Notch and JAK-STAT signaling through facilitating JAK2/STAT3 complex formation and subsequently promoting STAT3 phosphorylation and activation in the development of central nervous system.23 Considering the possible involvement of HES5 in the regulation of hepatic TPO generation (Figure 3D-E), we evaluated whether HES5 is capable of binding to JAK2 or STAT3 and plays a role in their activation in hepatocytes. We performed immunoprecipitation assays and found that desialylated platelet treatment promoted the interaction of HES5 with JAK2/STAT3 and phosphorylated JAK2/STAT3 (Figure 5A), indicating that HES5 binding to JAK2/STAT3 can stimulate STAT3 phosphorylation and activation, leading to hepatic TPO production.
HES5 binds to JAK2/STAT3 in hepatocytes. After treatment with desialylated platelets for 30 minutes, hepatocytes were lysed in NP-40 lysis buffer, followed by immunoprecipitation analysis of the relationship between JAK2/STAT3 and HES5 (representative images from 3 independent experiments). The binding relationship was quantified as a ratio to the respective input. Data were presented as mean ± SD (n = 3) and analyzed by 2-way ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
HES5 binds to JAK2/STAT3 in hepatocytes. After treatment with desialylated platelets for 30 minutes, hepatocytes were lysed in NP-40 lysis buffer, followed by immunoprecipitation analysis of the relationship between JAK2/STAT3 and HES5 (representative images from 3 independent experiments). The binding relationship was quantified as a ratio to the respective input. Data were presented as mean ± SD (n = 3) and analyzed by 2-way ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
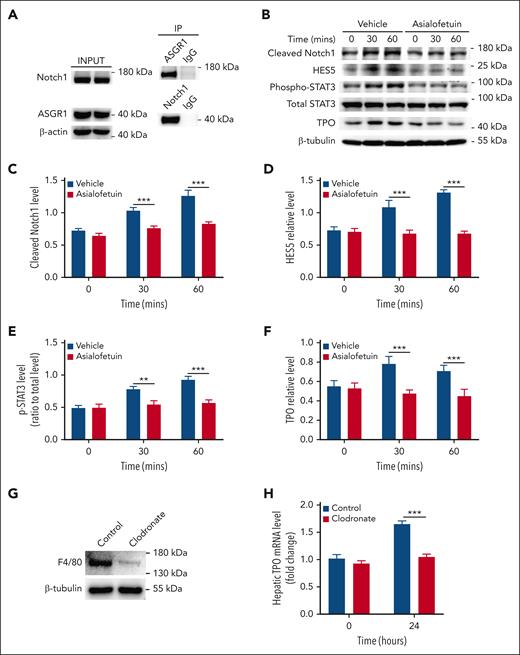
Association of Notch1 with ASGR1 in hepatocytes
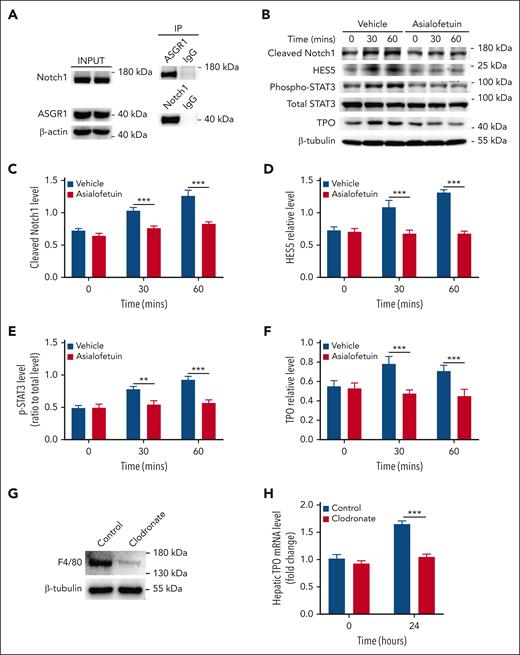
Under physiological conditions, desialylated platelets binding to hepatic AMR induces hepatic TPO expression.6 We measured the relationship between AMR and Notch1 by using immunoprecipitation assay and found that Notch1 was associated with the AMR subunit ASGR1 under resting condition (Figure 6A), indicating a physical proximity relationship between them.
Association of Notch1 with ASGR1 in hepatocytes. (A) The relationship between Notch1 and ASGR1 in hepatocytes were evaluated by immunoprecipitation assay (representative images from 3 independent experiments). (B) Hepatocytes were isolated from wild-type mice, incubated with Asialofetuin (200 μg/mL) for 15 minutes, and then treated with desialylated platelets, followed by measuring Notch1 activation (cleaved Notch1 expression) (C), HES5 expression (D), STAT3 phosphorylation (E), as well as TPO production (F) by western blot (data were shown as mean ± SD; n = 3; 2-way ANOVA). (G) Wild-type mice were IV injected with clodronate liposomes 2 days before infusion of desialylated platelets to deplete macrophage Kupffer cells, and the depletion efficiency was evaluated by measuring the expression of macrophage marker F4/80 in the liver by western blot (representative image was shown from 5 independent experiments). (H) Twenty-four hours after the transfusion of desialylated platelets, liver was isolated to measure TPO mRNA expression by quantitative real-time PCR (mean ± SE; n = 5; 2-way ANOVA). The expression of cleaved Notch1, HES5, and TPO was quantified as a ratio relative to β-tubulin level, and the phosphorylation level of STAT3 was quantified as a ratio relative to total level. Data were shown as mean ± SD (n = 3; 2-way ANOVA). ∗∗P < .01; ∗∗∗P < .001. PCR, polymerase chain reaction.
Association of Notch1 with ASGR1 in hepatocytes. (A) The relationship between Notch1 and ASGR1 in hepatocytes were evaluated by immunoprecipitation assay (representative images from 3 independent experiments). (B) Hepatocytes were isolated from wild-type mice, incubated with Asialofetuin (200 μg/mL) for 15 minutes, and then treated with desialylated platelets, followed by measuring Notch1 activation (cleaved Notch1 expression) (C), HES5 expression (D), STAT3 phosphorylation (E), as well as TPO production (F) by western blot (data were shown as mean ± SD; n = 3; 2-way ANOVA). (G) Wild-type mice were IV injected with clodronate liposomes 2 days before infusion of desialylated platelets to deplete macrophage Kupffer cells, and the depletion efficiency was evaluated by measuring the expression of macrophage marker F4/80 in the liver by western blot (representative image was shown from 5 independent experiments). (H) Twenty-four hours after the transfusion of desialylated platelets, liver was isolated to measure TPO mRNA expression by quantitative real-time PCR (mean ± SE; n = 5; 2-way ANOVA). The expression of cleaved Notch1, HES5, and TPO was quantified as a ratio relative to β-tubulin level, and the phosphorylation level of STAT3 was quantified as a ratio relative to total level. Data were shown as mean ± SD (n = 3; 2-way ANOVA). ∗∗P < .01; ∗∗∗P < .001. PCR, polymerase chain reaction.
Considering the involvement of AMR receptors in hepatic TPO production, we measured whether Notch1 deletion affected the expression of ASGR1 and ASGR2 in hepatocytes and found comparable expression levels between control and Notch1-deficient hepatocytes (supplemental Figure 13). We then investigated the role of AMR relative to Notch1 in hepatic TPO production using Asialofetuin or ASGR1 antibody to block AMR (Figure 6B) or ASGR1 (supplemental Figure 14), respectively, and found that inhibition of AMR or blocking ASGR1 significantly reduced Notch1 signaling activation (Figure 6C), HES5 expression (Figure 6D), STAT3 phosphorylation (Figure 6E), as well as TPO production (Figure 6F) in the isolated normal hepatocytes treated with desialylated platelets, indicating that the binding of desialylated platelets to hepatocyte AMR promotes Notch1 signaling activation, leading to upregulation of HES5 expression, which induces phosphorylation of JAK2/STAT3 and subsequent TPO production.
Liver Kupffer cells may express AMR.20,24 To evaluate the contribution of Kupffer cells to the desialylated platelet-induced hepatic TPO production, macrophages including liver Kupffer cells were depleted by clodronate treatment (Figure 6G), followed by administration of desialylated platelets. Our results showed that depletion of liver Kupffer cells significantly inhibited desialylated platelet-induced hepatic TPO production (Figure 6H). This is consistent with a previous study showing the requirement of Kupffer cells for platelet-mediated hepatic TPO generation25 and suggests that liver Kupffer cells participate in both clearance of desialylated platelets and hepatic TPO production. However, how the liver Kupffer cells, hepatocytes, and desialylated platelets coordinate this process to regulate hepatic TPO production remains to be investigated.
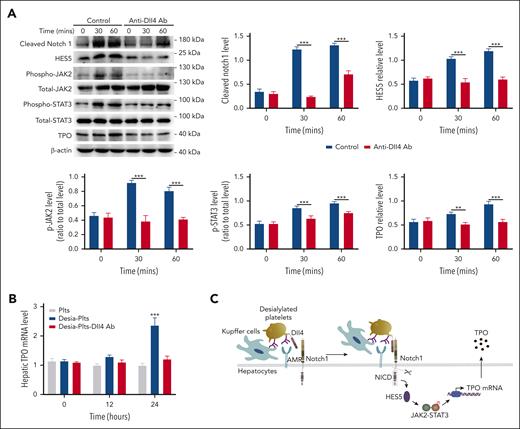
Binding of platelet Dll4 to hepatocyte Notch1 activates Notch1 signaling
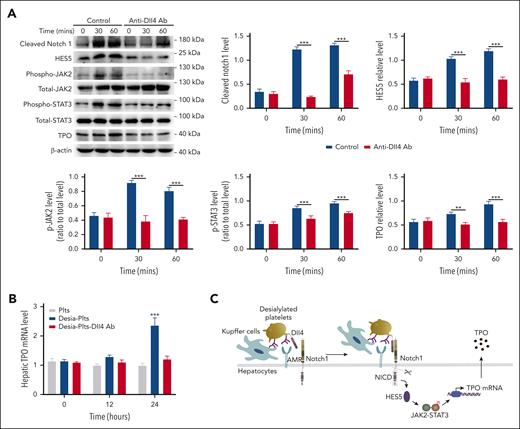
Notch1 signal transduction is triggered by the binding of a Notch1 ligand expressed on 1 cell to a Notch1 receptor on a neighboring cell.10,26 Because platelets express the Notch1 ligand Dll4,27 we hypothesized that binding of desialylated platelets to hepatic AMR would promote the binding of Dll4 on the surface of platelets to Notch1 on the surface of hepatocytes, given the proximal association of AMR with Notch1 (Figure 6A), resulting in Notch1 activation. Before testing this, we first evaluated whether desialylated platelets have increased Dll4 expression, and our results found no increase in Dll4 protein levels on the surface of platelets after 1 hour treatment with neuraminidase (supplemental Figure 15). We then pretreated desialylated platelets with a Dll4-blocking antibody, followed by incubation with hepatocytes. As seen in Figure 7, blocking Dll4 inhibited Notch1 activation, HES5 expression, JAK2/STAT3 phosphorylation, as well as TPO production (Figure 7A), indicating that engagement of platelet Dll4 with hepatocyte Notch1 activated Notch1 signal transduction, leading to HES5 upregulation, JAK2/STAT3 phosphorylation, and TPO production. To investigate the physiological role of platelet Dll4 in hepatic TPO production in vivo, desialylated platelets were treated with Dll4-blocking antibody before administration into wild-type mice, and the results showed a significant increase of hepatic TPO production after administration of control-treated desialylated platelets but not Dll4-blocked desialylated platelets (Figure 7B). In addition, blocking Dll4 also significantly inhibited hepatic Notch1 activation, HES5 expression, STAT3 phosphorylation, as well as TPO expression (supplemental Figure 16), further supporting the notion that engagement of Dll4 in desialylated platelets by hepatocyte Notch1 stimulates hepatic Notch1 signaling activation.
Inhibition of platelet Dll4 reduces hepatocyte Notch1 activation and TPO production. (A) Desialylated platelets were pretreated with Dll4 antibody (1 μg/mL) for 5 minutes and then incubated with hepatocytes for 30 or 60 minutes, followed by measuring Notch1 activation (cleaved Notch1 expression), HES5 expression, phosphorylation of JAK2/STAT3, and TPO level by western blot (mean ± SD; n = 3; 2-way ANOVA). The expression of cleaved Notch1, HES5, and TPO was quantified as a ratio relative to β-actin level, and the phosphorylation level of JAK2 and STAT3 was quantified as a ratio relative to respective total level. (B) Normal platelets (Plts), desialylated platelets (Desia-Plts), or desialylated platelets pretreated with Dll4 antibody (Desia-Plts-Dll4) (all at a total number of 2.5 × 108) were infused into wild-type mice, and at indicated time points, liver was isolated to measure hepatic TPO mRNA by quantitative PCR assay (mean ± SD; n = 3; 2-way ANOVA) (compared with Plts or Desia-Plts-Dll4 group, ∗∗∗P < .001). (C) Schematic outline of the role of Notch1 in the regulation of hepatic TPO production. PCR, polymerase chain reaction.
Inhibition of platelet Dll4 reduces hepatocyte Notch1 activation and TPO production. (A) Desialylated platelets were pretreated with Dll4 antibody (1 μg/mL) for 5 minutes and then incubated with hepatocytes for 30 or 60 minutes, followed by measuring Notch1 activation (cleaved Notch1 expression), HES5 expression, phosphorylation of JAK2/STAT3, and TPO level by western blot (mean ± SD; n = 3; 2-way ANOVA). The expression of cleaved Notch1, HES5, and TPO was quantified as a ratio relative to β-actin level, and the phosphorylation level of JAK2 and STAT3 was quantified as a ratio relative to respective total level. (B) Normal platelets (Plts), desialylated platelets (Desia-Plts), or desialylated platelets pretreated with Dll4 antibody (Desia-Plts-Dll4) (all at a total number of 2.5 × 108) were infused into wild-type mice, and at indicated time points, liver was isolated to measure hepatic TPO mRNA by quantitative PCR assay (mean ± SD; n = 3; 2-way ANOVA) (compared with Plts or Desia-Plts-Dll4 group, ∗∗∗P < .001). (C) Schematic outline of the role of Notch1 in the regulation of hepatic TPO production. PCR, polymerase chain reaction.
Discussion
TPO is mainly secreted from hepatocytes in the liver, although it can also come from other cells or tissues such as spleen or kidney. Under the physiological conditions, circulating TPO level is regulated by TPO receptors, which can bind to TPO, leading to internalization and destruction.4,5 Additionally, the aged desialylated platelets can also stimulate hepatic TPO production. In this study, using hepatocyte-specific Notch1 deletion mice, we showed that deficiency of hepatocyte Notch1 significantly reduces hepatic TPO production, leading to impaired megakaryocyte maturation and subsequent reduced number of circulating platelets, indicating a novel role of Notch1 in the regulation of hepatic TPO generation in a physiological way.
Notch signaling is a highly conserved network and plays fundamental roles in multiple developmental processes in both invertebrates and vertebrates.28 Several studies have demonstrated the critical roles of Notch signaling in the maturation and morphogenesis of the intrahepatic biliary system, with Notch2-Jag1 signaling axis playing fundamental role in the bile duct formation.15,29 In our study, hepatocyte-specific deletion of Notch1 does not affect liver ultrastructure and function, indicating the dispensable role of Notch1 in the liver development and function, consistent with a previous study showing no differences of bile duct structures and liver architecture between control mice and hepatocyte-specific Notch1 knockout mice.15 Additionally, their study also demonstrated no liver hyperplasia or enhanced hepatocytes proliferation in the knockout mice. However, in our study, hepatocyte Notch1-deficient mice presented a significantly lower number of platelets and white blood cells as well as TPO level. The exact mechanism by which hepatocyte Notch1 deletion affects white blood cells is unclear. Numbers of hematopoietic stem cells, common myeloid progenitors, and granulocyte/monocyte progenitors in BM from mice bearing hepatocyte Notch1 deletion were reduced relative to control mice, possibly due to the reduced level of TPO, a known regulator of hematopoiesis, hematopoietic stem cell self-renewal, and quiescence,30 resulting in a lower number of white blood cells. Moreover, Notch1 signaling is involved in leukocyte differentiation,31,32 and its deficiency in hepatocytes might reduce the level of some key downstream targets, which are required for the activation of Notch1 signaling pathway in the regulation of leukocyte differentiation, leading to reduced white blood cell count. Further studies are required to investigate this in the future. Given the thrombocytopenia in the knockout mice, the MPV value is in the normal range, which seems surprising. However, in the hepatocyte-specific Notch1 deficient mice, the thrombocytopenia is caused by the impaired platelet production (low TPO level) rather than the increased platelet clearance or abnormal platelet function (hyperactivity), indicating that the circulating platelets in the knockout mice do not have any abnormalities (with normal lifespan and function), supporting the normal morphology and MPV.
As a universal intracellular signaling pathway, JAK-STAT signaling network has been shown to participate in several cellular processes such as cell growth, proliferation, differentiation through regulation of the transcription, and expression of several target genes, thereby playing prominent roles in embryonic development, inflammation, and immune response.33,34 In the hematopoietic system, JAK-STAT signaling regulates the survival, proliferation, self-renewal, and differentiation of hematopoietic stem cells, thereby maintaining the hematopoietic hemostasis.35,36 Abnormal activation of JAK-STAT signaling has been demonstrated to be closely associated with several solid tumors33,37 and blood disorders,35,38 such as myeloproliferative neoplasms (characterized by an increased production of myelo-erythroid lineages), featured with a mutation of JAK2 (V617F), leading to constitutive activation of STAT3 and STAT5.39 In our study, we found a significantly reduced phosphorylation of JAK2 and STAT3 as well as TPO production in hepatocytes with Notch1 deletion, indicating the involvement of JAK2-STAT3 signaling in hepatic TPO production, consistent with a previous study showing that aged desialylated platelets stimulate hepatic TPO generation through JAK2-STAT3 signaling.6 The impaired activation of JAK2 and STAT3 in Notch1-deleted hepatocytes implies that Notch1 might play a role in the regulation of JAK2-STAT3 signaling, which is in accordance with a previous study showing the existence of a cross talk between Notch and JAK-STAT signaling in the development of central nervous system.23 Further analysis confirmed that HES5, a target gene of Notch1 signaling, mediates the cross talk through binding to JAK2/STAT3 and the phosphorylated form of JAK2/STAT3 in the hepatocytes, which is further supported by the reduced expression of HES5 in hepatocytes with Notch1 deletion.
The Notch signaling pathway is triggered or induced after the engagement of the ligands that are expressed on the surface of the neighboring cells. Currently, there are 5 identified ligands (Delta-like 1, Delta-like 3, Dll4, and Jagged-1/2).40 Although platelets do not have a nucleus, they have been reported to express Notch ligand Dll4.27 To investigate whether aged desialylated platelets activate hepatocyte Notch1 signaling through Dll4, we blocked desialylated platelets Dll4 using the blocking antibody and found that blocking of platelet Dll4 significantly inhibited Notch1 signaling activation, leading to reduced HES5 expression, JAK2/STAT3 phosphorylation, and subsequent TPO production in hepatocytes both in vitro and in vivo, indicating that platelet Dll4 activates hepatocyte Notch1 signaling. The AMR, originally named as hepatic ASGR, is a transmembrane GP complex that is composed of ASGR1 and ASGR2 subunits and involved in the recognition, binding, and clearance of asialoglycoproteins.41 The AMR endogenous ligands are identified as the desialylated prothrombotic components, such as platelets,42 and AMR can bind and remove the desialylated platelets.43,44 Considering the involvement of both AMR and Notch1 in the hepatic TPO production under physiological condition, their relationship was further investigated through the inhibition of AMR. Our results showed that blocking AMR significantly inhibited Notch1 signaling activation, JAK2/STAT3 phosphorylation, and TPO production in hepatocytes treated with desialylated platelets, indicating that binding of desialylated platelets to hepatocyte AMR as a capturing role facilitates the binding of desialylated platelet Dll4 to hepatocyte Notch1, given the proximal association of AMR and Notch1, leading to hepatocyte Notch1 signaling activation and subsequent hepatic TPO generation.
In conclusion, our study makes the novel finding that Notch1 and downstream pathways regulate hepatic TPO production (Figure 7C). Our work suggests that this pathway might be a novel avenue for targeted modulation of TPO levels.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grants 82322005, 82261138554, 82170130, 81970124, and 81400082), National Key Research and Development Program of China (grant 2023YFC2507800), the Natural Science Foundation of Jiangsu Province (grant BK20140219), funding for the Distinguished Professorship Program of Jiangsu Province, the Shuangchuang Project of Jiangsu Province, the 333 projects of Jiangsu Province (grant BRA2017542), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant 18KJA320010), Jiangsu Province's Graduate Scientific Research Innovation Program (grants KYCX23-2930, KYCX22-2896, and KYCX21-2691), Youth Science and Technology Innovation Team of Xuzhou Medical University, and the National Health and Medical Research Council of Australia.
Authorship
Contribution: Y.S., H.T., and X.C. performed research, analyzed data, and wrote the manuscript; Y.L., J.Z., Y.D., S.Z., X.G., C.C., M.X., and Z.L. performed research and analyzed data; E.E.G. and R.K.A. provided the intellectual input; L.Z., K.X., and J.Q. conceived and designed the study and wrote the manuscript; and all authors read and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jianlin Qiao, Blood Diseases Institute, Xuzhou Medical University, 84 West Huaihai Rd, Xuzhou 221002, China; email: jianlin.qiao@gmail.com.
References
Author notes
Y.S., H.T., and X.C. contributed equally to this study.
L.Z., K.X., and J.Q. are joint senior authors.
RNA-sequencing data of this article have been deposited in the Gene Expression Omnibus database (accession number GSE250529).
Original data are available on request from the corresponding author, Jianlin Qiao (jianlin.qiao@gmail.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Notch1 deficiency reduces the number of circulating platelet and TPO production. (A) Peripheral blood was drawn to measure the number of circulating platelets by an automatic blood analyzer (mean; n = 17; unpaired student t test). (B) Notch1fl/fl or HC Notch1−/− mice received intraperitoneal administration of rat anti-mouse integrin GPIIb/CD41 immunoglobulin at a dose of 0.1 mg/kg, followed by monitoring the number of circulating platelets at different time points (mean ± standard error of the mean [SE]; n = 7; 2-way analysis of variance [ANOVA]). Plasma (C) or liver mRNA (D) was isolated from Notch1fl/fl or HC Notch1−/− mice to measure TPO level by ELISA (mean ± SE; n = 3) or quantitative real-time PCR (mean ± SE; n = 8; unpaired student t test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/26/10.1182_blood.2023023559/3/m_blood_bld-2023-023559-gr1.jpeg?Expires=1768344427&Signature=aHc4je3PqD8ob3Us4~X8S~EZeO8Xx3pB~-sw~T2McMgvfMc8AIHiqejrZFXDK1KurlNq87iC6XxoUwtQimGcWabOHWPzqhwL14dG0l89PK0x-yhaVEbLmx3JWQvhAXM5dbzdpNdMLKRP0oeRXo7GE3EDuGZRFpmppasgFaYucG78M0zo~9k9W3qph6w3XEexYoDmixKM5ZwXGUjDIvBG6Ud4YwtzAGlcacT50Nz8B5mQSJPD7xAqZeYBWNKjLI1nd4L7ADUaV0Ti3LrWKth2YkQ3PYhp7I~CLh5R3TU-p4vZxuBJKkZJVNOtAHcaCvY9YRXQ7lK5uPNhtyscDrWorQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Reduced JAK2-STAT3 phosphorylation in Notch1-deficient hepatocytes. (A) The total RNA was isolated from liver of Notch1fl/fl mice or HC Notch1−/− mice and subjected into RNA-sequencing analysis. Differential gene expression between Notch1fl/fl and HC Notch1−/− group was analyzed and presented as volcano map. The x-axis shows the fold change, and the y-axis shows the P value. Red dots indicated the differentially upregulated genes with significance, and green dots presented the differentially downregulated genes with significance. (B) Signaling pathways enriched from KEGG pathway–based analysis. (C) Hepatocyte was isolated from Notch1fl/fl and HC Notch1−/− mice to measure the phosphorylation of JAK2/STAT3 and TPO level by western blot (mean ± standard deviation [SD]; n = 3; unpaired student t test). (D) Cultured hepatocytes were incubated with desialylated platelets for 30 minutes or 60 minutes, followed by measuring the phosphorylation of JAK2/STAT3, TPO level, Notch1 activation (cleaved Notch1 expression), and HES5 expression by western blot (mean ± SD; n = 3; 2-way ANOVA). (E) Expression of HES1 and HES5 in the hepatocyte of Notch1fl/fl and HC Notch1−/− mice was detected by western blot (mean ± SD; n = 3; unpaired student t test). The phosphorylation level of JAK2 and STAT3 was quantified as a ratio relative to respective total level, and the expression of TPO, HES5, HES1, and cleaved Notch1 was quantified as a ratio relative to β-actin level. ∗∗P < .01; ∗∗∗P < .001. ABC, ATP-binding cassette; PPAR, peroxisome proliferator-activated receptor; TGF-β, transforming growth factor-β.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/26/10.1182_blood.2023023559/3/m_blood_bld-2023-023559-gr3.jpeg?Expires=1768344427&Signature=w908D4X6GHHD08-FZjrrmuUbOP9dXgtpJVzGLXMu0DmA5PPbWSCeJinRhn6u0Qjm9Luuzr1u8KC7ynpl1nkNuwZT1ahPwCWqfDggnws3urjt6xVTiC0b4ijXJZTr0-9V6tJInWZ32ofqgdhZPyFZ139ukDuiz7kmdd9o6CN8HJHtnXj1yFAt9kw1-JtMi3-zOppUe4pQWlKBSrO-uD13WkgJg9sxIPh6JqxGtCu-2XoParfe-fa5cu9DZayY6F~5PcFD9irURo4Pmi6NJqpSe0G2vWzmECEF3wQ1-ZjQV~oXky-g9fVn-9LtULWh-LxXNHrbqlRYKaS6Chdv06WX-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Reduced JAK2-STAT3 phosphorylation in Notch1-deficient hepatocytes. (A) The total RNA was isolated from liver of Notch1fl/fl mice or HC Notch1−/− mice and subjected into RNA-sequencing analysis. Differential gene expression between Notch1fl/fl and HC Notch1−/− group was analyzed and presented as volcano map. The x-axis shows the fold change, and the y-axis shows the P value. Red dots indicated the differentially upregulated genes with significance, and green dots presented the differentially downregulated genes with significance. (B) Signaling pathways enriched from KEGG pathway–based analysis. (C) Hepatocyte was isolated from Notch1fl/fl and HC Notch1−/− mice to measure the phosphorylation of JAK2/STAT3 and TPO level by western blot (mean ± standard deviation [SD]; n = 3; unpaired student t test). (D) Cultured hepatocytes were incubated with desialylated platelets for 30 minutes or 60 minutes, followed by measuring the phosphorylation of JAK2/STAT3, TPO level, Notch1 activation (cleaved Notch1 expression), and HES5 expression by western blot (mean ± SD; n = 3; 2-way ANOVA). (E) Expression of HES1 and HES5 in the hepatocyte of Notch1fl/fl and HC Notch1−/− mice was detected by western blot (mean ± SD; n = 3; unpaired student t test). The phosphorylation level of JAK2 and STAT3 was quantified as a ratio relative to respective total level, and the expression of TPO, HES5, HES1, and cleaved Notch1 was quantified as a ratio relative to β-actin level. ∗∗P < .01; ∗∗∗P < .001. ABC, ATP-binding cassette; PPAR, peroxisome proliferator-activated receptor; TGF-β, transforming growth factor-β.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/26/10.1182_blood.2023023559/3/m_blood_bld-2023-023559-gr3.jpeg?Expires=1768344428&Signature=ySRnt-uyVN11S009svIsF8ncOUOEu8MY5hTbph0LcsS04ihUYkcwCDbW0TZF2~QiU0HOtTSsADiBQQzEJMNxs7yqwzFcypy19~N2EpJjCutME8S-Lgi398FVlX-sHr68r7lik5Kq50788adr2MS5Z17HgrJSiAsCiOmZzEipJ83qLUyoeeOjGkz2IJPORSHqDFTK1wldJ5VdobNBX3eDnpkqSsN~pdtodkU-uvy8zsuVW4iyvkspO68ZzA1uZvmzPxT8nD07Vm5JgqCT5x6yp~VJ7QEQDJEqzlT1TGKJKhc3qb~yWEQB1LG252dcm5fU856G9siWokdO3OUiypatRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)