Myelofibrosis is a type of myeloproliferative disorder, and research indicates that the immunosuppressive tumor microenvironment plays a significant role in the pathogenesis of myelofibrosis, with myeloid-derived suppressor cells (MDSCs) being the primary inhibitory cells involved in shaping the tumor immune microenvironment. In this study, we used flow cytometry to detect the number of MDSCs in the bone marrow of myelofibrosis patients and revealed the relationship between MDSCs and gene expression levels through single-cell transcriptome sequencing.
We utilized flow cytometry to identify the number of Lin-HLA-DR-CD33+ labeled MDSCs in the bone marrow of 56 myelofibrosis patients and 20 healthy individuals. Additionally, we performed single-cell transcriptome sequencing on samples from two myelofibrosis patients, one showing a favorable response to Lurcitanib treatment and the other showing an ineffective response.
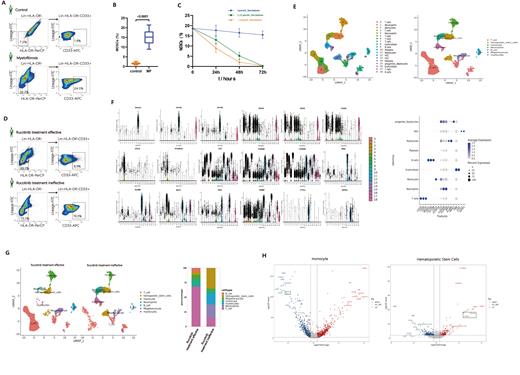
The results of flow cytometry revealed a significant increase in the percentage of MDSCs in the bone marrow of myelofibrosis patients (15.16±6.25%) compared to healthy individuals (1.72±0.88%) (p<0.0001) (Fig a, b). Among the two patients subjected to single-cell sequencing, the percentage of MDSCs in their samples was 19.3% and 8.9% (Fig c). Following treatment with different concentrations of decitabine, the MDSCs cell count progressively decreased with increasing treatment time and concentration (Fig d).Subsequently, we identified the major cell types in the two samples through single-cell sequencing, which included T cells, Neutrophils, Monocytes, Erythroblasts, B cells, Platelets, Myelocytes, Hematopoietic Stem Cells(HSCs), and progenitor Myelocytes (Fig e). The analysis of marker gene expression for each major cell type showed good specificity (Fig f). By comparing the proportions of different cell types between the two samples, it was observed that the high MDSC group had a decrease in the proportions of B cells, T cells, Monocytes, and Platelets, while an increase in the proportions of Neutrophils, Erythroblasts, HSC, Myelocytes, and progenitor Myelocytes (Fig g).Furthermore, we analyzed the highly differentially expressed genes in different cell types and identified significantly enriched pathways. The results showed significant downregulation of the GPX1 gene in monocytes of the high MDSC group, whereas the S100A8/S100A9 genes were significantly upregulated in HSC (Fig h). Enrichment analysis revealed significant enrichment of pathways related to reactive oxygen species metabolism, immune-inflammatory response, etc., in monocytes of the high MDSCs group, and significant enrichment of pathways related to NF-kappaB transcription factor activity regulation, Jak-STATs signaling, mTOR signaling in HSC.
Research has shown that the number of MDSCs is elevated in patients with myelofibrosis . Lurcitanib exhibits poor therapeutic efficacy in myelofibrosis patients with high MDSCs counts, whereas decitabine demonstrates more favorable treatment outcomes. Single-cell sequencing results revealed significant downregulation of the GPX1 gene in monocytes of myelofibrosis patients with high MDSCs counts. GPX1 gene encodes glutathione peroxidase 1, which plays a role in reducing oxidative stress and inflammation, and has anti-fibrotic effects.Conversely, in HSC of myelofibrosis patients with high MDSCs counts, the S100A8/S100A9 gene expression is significantly upregulated. This gene can induce neutrophil chemotaxis and adhesion, serving as a danger-associated molecular pattern (DAMP) molecule and stimulating innate immune cells through pattern recognition receptors (e.g., TLR4 and AGER), subsequently activating MAP kinase and NF-kappa-B signaling pathways, leading to inflammation and fibrosis.Thus, it can be inferred that the number of MDSCs in myelofibrosis patients is negatively correlated with GPX1 gene expression and positively correlated with S100A8/S100A9 gene expression.
Acknowledgement: This research was funded by the Key R&D Program of Zhejiang, No. 2022C03137; Public Technology Application Research Program of Zhejiang, China, No. LGF21H080003; Zhejiang Medical Association Clinical Medical Research special fund project, No. 2022ZYC-D09.
Correspondence to: Dr Jian Huang, Department of Hematology, The First Affiliated Hospital of Zhejiang University School of Medicine. househuang@zju.edu.cn
Disclosures
No relevant conflicts of interest to declare.