Introduction: Owing to comorbidities and poor tolerance to standard-dose chemotherapy, elderly patients with newly diagnosed diffuse large-B cell lymphoma (DLBCL) have to reduce their chemotherapy dose, ultimately leading to a poor prognosis (Di M, et al. Oncologist, 2021). Therefore, new therapeutic strategies with superior efficacy and less toxicity are urgently needed. The Smart Start study demonstrated that induction therapy with rituximab (R), lenalidomide, and ibrutinib resulted in a high overall response rate (ORR) in patients with newly diagnosed DLBCL (Westin J, et al. J Clin Oncol, 2023). Orelabrutinib (O), as a novel covalent Bruton's kinase inhibitor (BTKi) with high target selection, was reported to preserve the NK-cell-mediated antibody-dependent cellular cytotoxicity induced by R and thus boosted the antitumor effect of R-based regimen (Yu H, et al. Mol Ther-Oncolytics, 2021). The responders to OR induction therapy have been reported to attain synergistic antitumor effect and high complete remission (CR) rate (CRR) when receiving subsequent treatment (Qu C, et al. Hematol Oncol, 2023). Here, a prospective study was conducted to investigate the efficacy and safety of pomalidomide (P; a third-generation immunomodulatory drug), R, O, and miniCHOP-like (PRO-miniCHOP) in elderly patients with newly diagnosed DLBCL.
Methods: Patients aged ≥70 years with newly diagnosed DLBCLwere enrolled in this open-label, single-arm, phase II study (NCT05809180). All eligible patients received one 21-day cycle of induction therapy with PRO (P, 4 mg, d1-7; R, 375 mg/m 2, d1; O, 150 mg, QD). Subsequently, patients who achieved at least mini response (miniR, a reduction in tumor lesions by 25%-50%) were administered additional 6 cycles of PRO-miniCHOP regimen (PRO with reduced-dose CHOP regimen [cyclophosphamide, 400 mg/m 2, d2; doxorubicin/liposomal doxorubicin, 25 mg/m 2/15 mg/m 2, d2; vindesine, 2 mg, d2; and dexamethasone, 7.5 mg/m 2, d2-6]). The subsequent treatment was administrated according to the tumor response after 6 cycles of the PRO-miniCHOP regimen, including the end of treatment for CR, 2 years of pomalidomide maintenance therapy for partial remission (PR), and discharge from the study for stable disease or progression disease. The primary endpoints were ORR and CRR after 6 cycles of the PRO-miniCHOP regimen. Secondary endpoints were ORR and CRR at the end of the induction therapy, as well as 2-year progression-free survival, 2-year overall survival and safety.
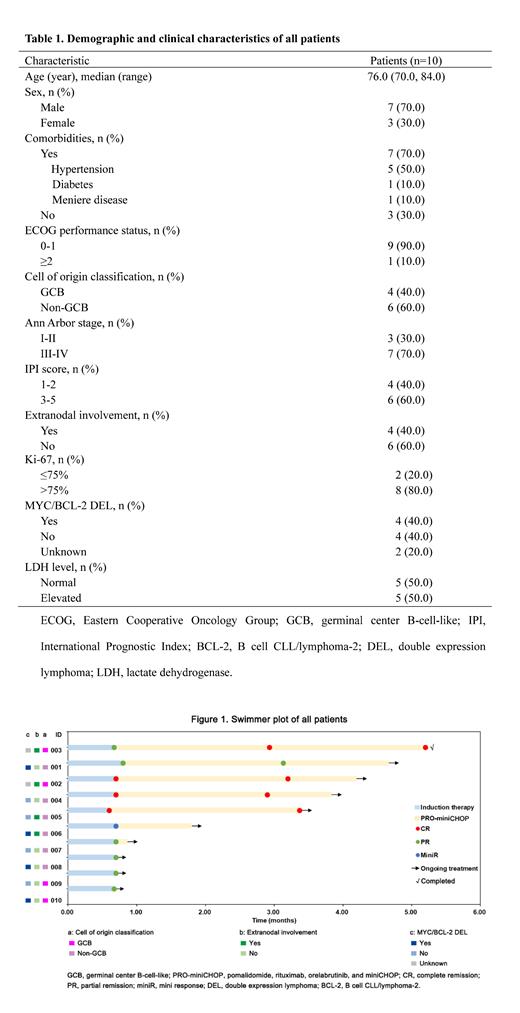
Results: From January 01, 2023 to July 30, 2023, 10 patients were enrolled in this study. The median age was 76.0 (range, 70.0-84.0) years (Table 1). The majority of patients had an Ann Arbor stage of III or IV (7/10, 70.0%), International Prognostic Index score of ≥3 (6/10, 60.0%), and non-germinal center B-cell-like subtype (6/10, 60.0%). Five (50.0%) patients had hypertension, 4 (40.0%) patients presented with extranodal involvement, and MYC/BCL-2 double expression lymphoma (40.0%). After one cycle of induction therapy with PRO, all (10/10, 100.0%) patients achieved at least miniR, including 3 CR, 6 PR, and 1 miniR (Figure 1). Five patients completed ≥3 cycles of the PRO-miniCHOP regimen, among whom 4 (80.0%) achieved a CR and 1 (20.0%) had a PR, with the best ORR of 100%; of these, one sustained CR at the end of cycle 6. During the induction therapy with PRO, 50.0% (5/10) of patients reported adverse events (AEs) of any grade, the majority of which were grade 1-2, with only 2 patients experiencing grade 3-4 neutropenia. Grade ≥3 AEs during the whole treatment period were neutropenia (6/10, 60.0%), thrombocytopenia (1/10, 10.0%), and lymphopenia (1/10, 10.0%), of which one (1/10, 10.0%) patient developed febrile neutropenia. Notably, the use of prophylactic pegylated recombinant human granulocyte colony stimulating factor significantly reduced the recurrence rate of severe neutropenia in the subsequent treatment, and no treatment-associated death was observed. Moreover, despite half of the patients having the comorbidity of hypertension, no off-target related cardiac events such as atrial fibrillation or atrial flutter were observed. At the time of data cutoff, 9 patients were still under treatment.
Conclusions: The present study provided preliminary evidence supporting the use of the PRO-miniCHOP regimen in elderly patients with newly diagnosed DLBCL. More clinical data will be updated from this ongoing study.
Disclosures
No relevant conflicts of interest to declare.