Congenital fibrinogen disorders (CFDs) are rare and make up approximately 0.6% of all inherited bleeding disorders. Two subtypes include a quantitative deficiency of fibrinogen, congenital afibrinogenemia (CA), congenital hypofibrinogenemia (CH), and qualitative defect of the fibrinogen molecule, congenital dysfibrinogenemia (CD). Patients with CD may have a thrombotic as well as a hemorrhagic disease phenotype. In the past, fresh frozen plasma or cryoprecipitate were the only options available for treatment and prevention of bleeding in patients with CFD. Today in the US, human plasma-derived concentrates (FIBRYGA, Octapharma, Lachlen, Switzerland, and RiaSTAP, CSL, Melbourne, Australia) are available and considered safe and effective treatment options for CD and CH. Guidelines for the treatment and prevention of bleeding is not established for CFDs, and especially for CD.
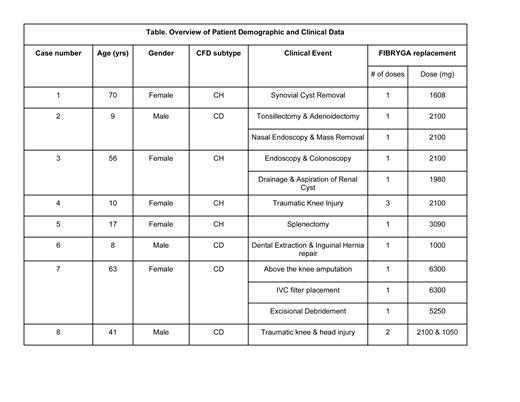
We present eight clinical cases of patients with hypo- or dysfibrinogenemia that were effectively treated with a pooled, plasma-derived fibrinogen concentrate (FIBRYGA) either prophylactically for elective or urgent surgery, or post-traumatic injury. The mean age of patients treated was 34 years (min-max 8-70 years). Three patients were male and five, female. Four patients had CH and four, CD. FIBRYGA was used to prophylactically treat a total of ten surgical procedures and two post-traumatic events in these patients. Dosing of FIBRYGA was based on the goal peak plasma fibrinogen concentration and the manufacturer's prescribing information, but modified to align with local laboratory reference ranges for the functional fibrinogen test. No acute or delayed infusion reactions (nausea, fever, rigors, headache) and no hypersensitivity reactions were noted. One patient developed two adverse events, one thrombotic and one hemorrhagic. This patient underwent left, above the knee amputation (AKA) and had a complicated postoperative period due to her multiple comorbidities including type 2 diabetes, hypertension, and long standing, poorly-controlled, severe peripheral arterial disease. She presented nineteen days after receiving FIBRGYA with a PE and multiple DVTs. Fifty-nine days later she presented with wound dehiscence and underwent an extensive excisional debridement with postoperative bleeding deemed to be secondary to her strap sliding during surgery and acting as a tourniquet for venous blood flow.
For the majority (86%) of our patients described here with CH and CD, FIBRYGA was well tolerated and effective in preventing post procedural or post traumatic bleeding complications. The post procedural bleeding noted in one patient may have been due to inadequate fibrinogen concentration or other comorbid conditions. Further clinical studies are needed to determine the role of FIBRGYA and other HFC in the treatment of patients with CFD, especially those with CD.
OffLabel Disclosure:
Tarantino:Takeda: Other: Clinical trial investigator, Research Funding; Amgen: Consultancy; Biomarin: Consultancy; Genentech: Consultancy; Novartis: Consultancy; Octapharma: Consultancy, Other: Clinical trial investigator; Principia: Consultancy; Spark: Other: Clinical trial investigator. Ansteatt:Genentech: Consultancy; Novartis: Consultancy; Sobi: Consultancy.
Fibryga, fibrinogen concentrate, currently approved for patients with afibrinogenemia and hypofibrinogenemia but not approved for persons with dysfibrinogenemia. This abstract will describe the use of Fibryga for persons with dysfibrinogenemia and hypofibrinogenemia.