Introduction:
Therapy related Myelodysplastic Syndrome and Acute Myeloid Leukemia (t-MDS/AML) is a well-established adverse event (AE) associated with conventional chemotherapy drugs and has historically carried a poorer prognosis when compared to de novo disease. Newer chemo medicines like PARP inhibitors have also been implicated in developing this complication. However, comprehensive real-world data is lacking pertaining to the AE burden relative to individual drug classes.
Methods:
We used the FDA Adverse Events Report System (FAERS) public dashboard to obtain the data. Data was available for the years 1970 - 2023. We retrieved the number of events that were reported as “Myelodysplastic Syndrome” or “Acute Myeloid Leukemia” for individual chemotherapy classes that have historically been known to cause t-MDS/AML. Disproportionality analysis was performed by calculating the reporting odds ratio (ROR) with its 95% confidence interval (CI).
Results:
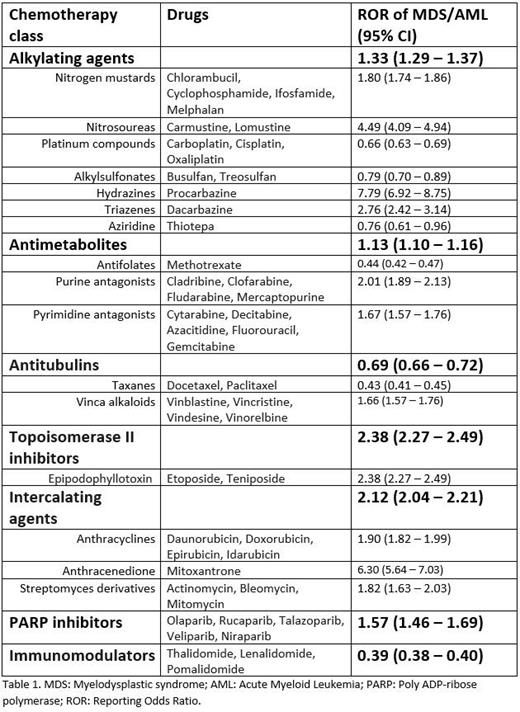
The drug class with the highest ROR of MDS/AML was the topoisomerase inhibitors - ROR 2.38 (95% CI 2.27-2.49). Intercalating agents like anthracyclines and mitoxantrone had the next highest ROR of 2.12 (95% CI 2.04 - 2.21). The novel drug class PARP inhibitors had the third most burden of t-MDS/AML with an ROR of 1.57 (95% CI 1.46 - 1.69). Table 1. depicts the individual drug classes and their ROR for associated MDS/AML. Table 1 depicts the individual drug classes and their ROR for associated MDS/AML.
Conclusions:
From this retrospective analysis, we identified that among all the chemotherapy drug classes, the topoisomerase II inhibitors were associated with the highest burden of t-MDS/AML, followed by intercalating agents and PARP inhibitors. Further data is essential to identify the patient characteristics associated with higher risk of developing this dreaded complication. This could improve patient-counseling prior to treatment initiation, assist with alternate drug choices when applicable, and perhaps also aid in developing strategies for closer monitoring in high-risk patients.
Disclosures
No relevant conflicts of interest to declare.