Background:
Despite clinical outcome improvements observed in relapsed/refractory (R/R) B-cell lymphoma patients (pts) treated with antigen receptor modified T-cells (CAR-T cells), a sizeable proportion still progress or relapse after infusion. Besides, this procedure is associated with significant morbidity primarily due to the immune effector cell-associated neurotoxicity syndrome (ICANS). The main goal of the CART-AI-Radiomics study is to apply artificial intelligence to develop new imaging-based prognostic and predictive models that integrate molecular and imaging biomarkers towards improved stratification of pts at high risk of R/R. In this sub-study, we retrospectively assessed the ability of imaging features and clinical data to predict survival and neurotoxicity.
Methods:
Consecutive pts diagnosed of diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma (PMBCL) treated with anti-CD19 CAR-T cells from 2019-2022 in Valencia Clinic Hospital and La Fe Hospital were included (cut-off date: July 2023). All the pre-infusion flourine18 fluorodeoxyglucose positron emission tomography/computed tomography ( 18F-FDG PET/CT) scans and clinical data were collected. The volume of interest (VOI) was manually contoured for all visible lesions in the PET/CT imaging exams. A subsequent imaging features extraction was performed. Features included PET conventional parameters-maximum standardized uptake value (SUV max), metabolic tumor volume (MTV; defined as the volume of voxels with SUVs higher than the threshold of 41% × SUV max) and total lesion glycolysis (TLG)-and radiomic features. Imaging parameters were calculated for all lesions (total) and for the dominant lesion (DL), whereas clinical variables were measured before lymphodepletion (LD). Univariate and multivariate analyses were carried out using Cox proportional-hazards models for survival prediction and logistic regression for neurotoxicity assessment. Survival analyses were conducted according to MTV total values.
Results:
A total of 29 pts (median age 63.2 [range 21.8-79.3] years; 18 [62.1%] females) who received CAR-T cell therapy were included. Twenty-six (89.7%) pts had DLBCL and 3 (10.3%) had PMBCL. At the time of pre-LD, 22 (75.9%) pts had increased lactate dehydrogenase (LDH) levels 18 (62.1%), presented with stage IV disease, 16 (55.17%) with an International Prognostic Index (IPI) score of 3-5, 8 (27.6%) had bulky disease. Median number of prior treatment lines was 2 (range 2-6). Twenty-three (79.3%) pts were treated with axicabtagene ciloleucel and 6 (20.7%) with tisagenlecleucel. Bridging therapy before LD was required by 26 (89.6%) pts.
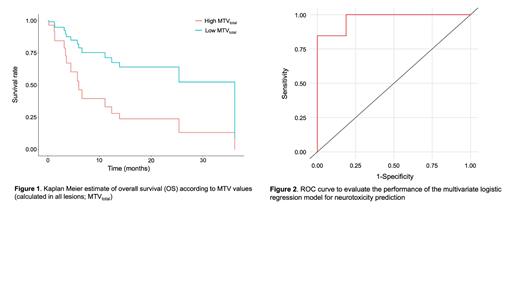
For survival analyses, a cut-off MTV total value of 236.03 mL that maximized the Log-Rank statistic was selected to divide pts into high MTV total and low MTV total categories. Median overall survival (OS) was 5.7 [3.2-NA, 95% CI] months and 36.2 [13.9-NA, 95% CI] months for the high and low MTV total, respectively (Fig. 1). In multivariate analyses, a model including MTV total, SUV max and TLG total as imaging feature, and ICANS, C-reactive protein LD (CRP LD), LDH LD, lymphocytes count LD (Li LD) as clinical variables was able to successfully predict OS ( p = 0.019). Median progression-free survival (PFS) was 3 [1.31-NA, 95% CI] months and 11.06 [5.81-NA, 95% CI] months for the high and low MTV total, respectively. In multivariate analysis, a successful prediction of PFS ( p = 0.007) was achieved with a model including the same predictors that were used for OS.
For the neurotoxicity assessment, a logistic regression model including six conventional PET parameters-MTV total, MTV DL,TLG total, TLG DL, median SUV DL, SUV max-two radiomic features,-entropy and entropy DL-and four clinical variables-CRP LD, LDH LD, Li LD and type of CAR-T cell treatment-was able to successfully predict the development of ICANS with an area under the curve of 0.971, a sensitivity of 0.813, a specificity of 0.846 and an accuracy of 0.828 (Fig. 2).
Conclusions:
Imaging features extracted from pre-infusion 18F-FDG PET/CT images in combination with several clinical features could predict survival and neurotoxicity in pts with DLBCL or PMBCL treated with CAR-T cell therapy. Quantitative features extracted from the PET/CT imaging exams may be useful for patient risk stratification and neurotoxicity prediction. Validation of these classifiers in independent datasets is warranted.
Disclosures
Hernandez Boluda:Pfizer, BMS, Incyte, and Novartis: Membership on an entity's Board of Directors or advisory committees. Guerreiro:Novartis, Kite, BMS, MSD, Pierre Fabre: Consultancy; IIS La Fe: Current Employment. Fuster-Matanzo:QUIBIM: Current Employment. Picó:QUIBIM: Current Employment. Estepa-Fernández:QUIBIM: Current Employment. Fernández:QUIBIM: Current Employment. Bellvís-Bataller:QUIBIM: Current Employment. Weiss:QUIBIM: Current Employment. Terol:Hematologist, Head of the lymphoma Unit, Department of Hematology, Insitute of Research INCLIVA, University of Valencia, Spain: Current Employment; Beigene, Gilead, F. Hoffmann-La Roche Ltd, Abbvie, Janssen: Consultancy; Gilead: Research Funding; F. Hoffmann-La Roche Ltd, Janssen, Gilead, Takeda, Abbvie, Beigene: Speakers Bureau; F. Hoffmann-La Roche Ltd, Janssen, Gilead, Takeda, Abbvie, Beigene: Membership on an entity's Board of Directors or advisory committees.