Background: The TIGER-trial (NCT01657604) is a multicenter, randomized phase III study to evaluate efficacy and tolerability of nilotinib (NIL) vs NIL+pegylated interferon alpha2b (IFN) combination therapy with IFN maintenance as first-line treatment for patients (pts) with chronic myeloid leukemia (CML) in chronic phase.
Methods: A total of 717 pts were recruited from 110 sites in Germany, Switzerland, and the Czech Republic. A pilot phase (n=25) validated the feasibility of the combination of NIL 300 mg BID and IFN (30-50µg/week according to tolerability and commenced after ≥6 weeks NIL monotherapy). In the main phase, 692 pts were randomly assigned to NIL (n=353) and NIL/IFN (n=339). Achievement of major molecular remission (MMR, BCR::ABL1 ≤0.1% on the International Scale, IS) after >24 months (mo) of therapy was the trigger to start the maintenance phase; treatment-free remission (TFR) started in pts with ≥12 mo persistence of MR 4 ( BCR::ABL1 ≤0.01% IS) after >36 mo total therapy. Quality of life (QoL) was evaluated using EORTC QLQ-C30 and CML24 questionnaires.
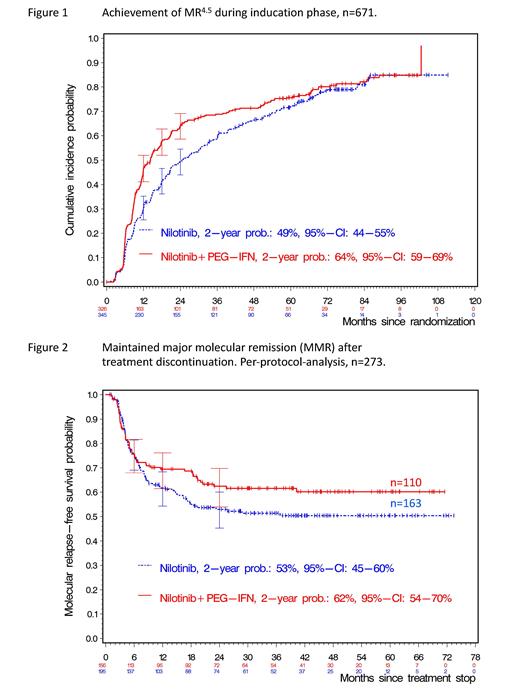
Results: From 692 randomized pts, 411 were male (59%), median age was 51 years (range, 18-85). In the monotherapy arm, median treatment duration with NIL was 3.1 years (0.02-8.9), median daily dose 600 mg (183-764). In the combination arm, median treatment duration with NIL was 2.3 years (0.02-9.1), median daily dose 600 mg (106-792). Median duration of IFN therapy was 2.4 years (0-9.0). A median of 77 (0-485) IFN injections were administered, the median dose of IFN per injection was 30µg (0-50). Probabilities of MMR and MR 4.5 ( BCR::ABL1 ≤0.0032% IS, Fig 1) by 24 mo were 89% (95% CI: 85-92%) and 49% (44-55%) vs 93% (89-95%) and 64% (59-69%) with NIL vs NIL/IFN, respectively. In 356 pts (53%) qualifying for the discontinuation phase (NIL, n=197; NIL/IFN, n=159), probabilities of maintained MMR by 24 mo were 53% (45-60%) and 62% (54-70%) after NIL and NIL/IFN, respectively, in an intention-to-treat-analysis (p=0.13). 273 (40%) eligible pts actually discontinued therapy (per-protocol-analysis, NIL, n=163; NIL/IFN, n=110). Probabilities of TFR by 24 mo were 53% (45-61%) vs 59% (49-68%) for NIL and NIL/IFN, respectively (Fig 2).
Fifteen pts (2.2%) with atypical BCR::ABL1 transcripts (e1a2, n=7; e19a2, n=4; e8a2, n=2; e13a3 and e14a3, n=1 each) were randomized to receive NIL (n=7) or NIL/IFN (n=8). After a median treatment period of 37 mo (36-39) 9 pts achieved and maintained a BCR::ABL1 reduction of at least 4 orders of magnitude and were eligible for TFR. 6 pts failed to achieve an individual transcript decline by at least 3 logs. TFR was commenced in 7 and maintained in 6 pts after 32 (range, 20-84) mo.
Adverse events of special interest grades 3-5 were arterio-vascular disorders in 9 vs 8%, fatigue in 2 vs 4%, thrombocytopenia in 8 vs 8%, and alanine aminotransferase elevation in 4 vs 9% of pts in the NIL vs NIL/IFN arms, respectively. QoL analyses revealed the perception of a decreased cognitive function and higher rates of fatigue in pts in the NIL/IFN arm, particularly in pts older than 40 years.
In total, 24 pts (NIL, n=13; NIL/IFN, n=11) progressed to advanced disease. By 8 years, progression-free survival was 94% (95% CI: 90-96%) and 92% (88-95%), overall survival 95% (92-97%) and 94% (91-97%) in the NIL and NIL/IFN arms, respectively. 28 pts (3.9%) received an allogeneic stem cell transplantation, 14 after disease progression. 35 pts died (NIL, n=18; NIL/IFN, n=17), 9 related to CML.
Conclusions: Survival of CML pts has reached probabilities close to normal. The combination of NIL with IFN is associated with a higher rate of molecular responses but also impaired tolerability. IFN maintenance is feasible, and resulted in a trend towards higher rates of long-term TFR.
The study was conducted on behalf of the German CML Study Group in cooperation with the East German Study Group on Hematology and Oncology (OSHO) and the Swiss Group for Clinical Cancer Research (SAKK).
OffLabel Disclosure:
Hochhaus:Bristol Myers Squibb: Consultancy, Research Funding; Incyte: Research Funding; Pfizer: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Burchert:MSD: Research Funding; Incyte: Honoraria; Novartis: Honoraria, Research Funding. Saussele:Incyte: Research Funding; BMS: Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Roche: Consultancy. Baerlocher:MSD: Research Funding; Novartis: Research Funding; Geron Corporation: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; GSK: Consultancy, Honoraria. Mayer:MSD: Research Funding; Novartis: Research Funding. Brümmendorf:Janssen: Consultancy, Honoraria, Speakers Bureau; Merck: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau. La Rosée:MSD: Research Funding; Novartis: Research Funding. le Coutre:Incyte: Honoraria; Blueprint: Honoraria; AOP: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Novartis: Honoraria. Hanel:Novartis: Research Funding. Stegelmann:AOP Pharma: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; GSK: Consultancy, Honoraria. Radsak:Cogent Biosciences: Honoraria; Pfizer: Honoraria; JAZZ: Other: Travel support; Bristol Myers Squibb: Other: Travel support; TEVA: Honoraria; Abbvie: Honoraria; Lilly: Honoraria; Otsuka: Honoraria; Takeda: Honoraria; Novartis: Other: Travel support; Corat: Honoraria; Incyte: Honoraria; Daiichi Sankyo: Other: Travel support; Glaxo Smith Kline: Honoraria; Beigene: Honoraria; Novartis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Amgen: Other: Travel support; Abbvie: Other: Travel support; Astellas: Other: Travel support; SOBI: Other: Travel support; AOP: Other: Travel support. Kunzmann:Novartis: Research Funding. Zackova:Pfizer: Honoraria, Other: travel grant, Speakers Bureau; Astra Zeneca: Other: travel grant; Angelini Pharma: Consultancy, Honoraria, Other: travel grant, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grant, Speakers Bureau. Ernst:Novartis: Research Funding. Pfirrmann:Novartis: Honoraria.
Combination of Nilotinib and pegylated Interferon alpha in CML.