Background: Recurrent joint bleeding from a young age in people with hemophilia A (PwHA) may lead to hemophilic arthropathy, resulting in limited movement and chronic pain. Emicizumab is a bispecific monoclonal antibody that bridges activated factor (F) IX and FX to substitute for activated FVIII and provides stable plasma concentrations and maintenance of trough levels within the therapeutic range. Limitations in long-term joint health preservation have been reported with FVIII prophylaxis (Warren et al. Blood Adv 2020); it is expected that emicizumab may overcome these limitations, as joint health improvements have been demonstrated in PwHA aged 12-39 years (Kiialainen et al. Haemophilia 2022). Here, we report the 3-year interim analysis of the ongoing, open-label, Phase IV AOZORA study (JapicCTI-194701), analyzing long-term safety and joint health in pediatric PwHA without FVIII inhibitors receiving emicizumab (Shima et al. BMJ Open 2022).
Methods: Eligible participants were aged <12 years with severe hemophilia A (HA) without FVIII inhibitors. Participants entered AOZORA as emicizumab-naïve or following the Phase III HOHOEMI study (Shima et al. Haemophilia 2019). Primary endpoints include adverse events (AEs), and joint health and function assessed by magnetic resonance imaging (MRI) and the Hemophilia Joint Health Score (HJHS) version 2.1. MRI evaluation uses the International Prophylaxis Study Group (IPSG) MRI scale; a positive IPSG score indicates pathological changes in the knee and/or ankle joints. Exploratory endpoints include annualized bleed rate (ABR) for treated bleeds and treated joint bleeds. All participants will receive emicizumab for 6 years from their first dose until study end.
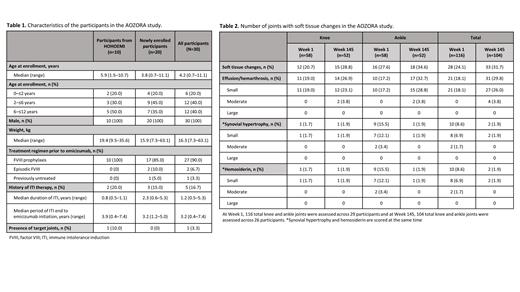
Results: Data cut-off was the last day of Week 145 for individual participants. Ten participants entered from HOHOEMI and 20 participants were newly enrolled. The median (range) age was 4.2 (0.7-11.1) years, all participants were male, and 27 (93.1%) participants had been treated with prior FVIII prophylaxis ( Table 1). One participant had 1 target joint (knee) at Week 1. In AOZORA, 5 (16.7%) participants experienced 7 emicizumab-related AEs: 6 injection site reactions in 5 participants and 1 case of anemia in 1 participant. Seven serious AEs, reported as unrelated to emicizumab, occurred in 5 (16.7%) participants. No thrombotic events or thrombotic microangiopathies occurred. Twenty-nine participants at Week 1 and 26 participants at Week 145 were assessed using the IPSG MRI scale. At Week 145, MRI scores remained at 0 for 11 (42.3%) of the 26 participants. Of 116 joints assessed at Week 1, 28 (24.1%) had soft tissue changes (effusion/hemarthrosis, synovial hypertrophy and/or hemosiderin deposition; Table 2). At Week 145, 104 joints were assessed and 33 (31.7%) of those had soft tissue changes. Of 10 (8.6%) joints with synovial hypertrophy and hemosiderin at Week 1, all had improved at Week 145 and synovial hypertrophy and hemosiderin were no longer present in 9 joints. One new joint developed synovial hypertrophy and hemosiderin at Week 145. The rest of the soft tissue changes were effusion/hemarthrosis, present in 21 (18.1%) joints at Week 1 and 31 (29.8%) at Week 145. Thirty participants at Week 1 and 27 participants at Week 145 were assessed using the HJHS v2.1. Eighteen (66.7%) participants' HJHS scores remained at 0 from Week 1 to Week 145 and 1 (3.7%) participant's remained at 4; 6 (22.2%) participants' scores improved; 2 (7.4%) participants' scores worsened. Mean (standard deviation) HJHS total score improved from 0.90 (1.79) at Week 1 to 0.44 (1.05) at Week 145. The mean (95% confidence interval [CI]) ABR for treated bleeds was 3.7 (0.94-9.80) prior to emicizumab and 0.7 (0.01-5.10) while receiving emicizumab. The mean (95% CI) ABR for treated joint bleeds was 0.4 (0.00-4.55) prior to emicizumab and 0.2 (0.00-4.04) while receiving emicizumab.
Conclusions: This 3-year interim analysis of the AOZORA study confirmed the safety profile of emicizumab. The proportion of joints with effusion/hemarthrosis increased by Week 145. However, all joints with synovial hypertrophy and hemosiderin, which are strongly associated with the progression of arthropathy (Gualtierotti et al. J Thromb Haemost 2021), improved by Week 145. These results suggest that emicizumab is effective in maintaining and improving joint health in pediatric PwHA.
Disclosures
Shima:Nara Medical University: Current Employment; Chugai Pharmaceutical Co., Ltd, Fujimoto Seiyaku, KYorin, Pfizer: Consultancy; Takeda, CSL Behring: Research Funding; Chugai Pharmaceutical Co., Ltd, CSL Behring, Sanofi, Novo Nordisk: Speakers Bureau. Takedani:Bayer, Chugai Pharmaceutical Co., Ltd, CSL Behring, Sanofi, Novo Nordisk, KMB: Speakers Bureau; Bayer, Chugai Pharmaceutical Co., Ltd, CSL Behring, Novo Nordisk: Honoraria. Kitsukawa:Chiba University Hospital: Current Employment; CSL Limited, Chugai Pharmaceutical Co., Ltd: Honoraria. Taki:Chugai Pharmaceutical Co., Ltd, Bayer, KM biologics, Fujimoto, Takeda, Sanofi, Novo Nordisk, Pfizer, CSL Behring: Honoraria. Ishiguro:National Center for Child Health and Development: Current Employment; Chugai Pharmaceutical Co., Ltd, Pfizer: Research Funding. Nagae:Chugai Pharmaceutical Co., Ltd., CSL Behring, Novo Nordisk, Sanofi, Takeda: Speakers Bureau. Nagao:Sanofi K.K., Takeda, Chugai Pharmaceutical Co., Ltd., Bayer Holding Ltd., Fujimoto Pharmaceutical Corporation, KM Biologics, Pfizer Japan Inc., Japan Blood Products Organization, Novo Nordisk, Sekisui Medical Co., Ltd., CSL Behring: Honoraria; Takeda: Consultancy; Bayer Holding Ltd., Takeda, Chugai Pharmaceutical Co., Ltd for non- related study: Research Funding. Nosaka:Chugai Pharmaceutical Co., Ltd: Current Employment. Kyogoku:Chugai Pharmaceutical Co., Ltd: Current Employment. Oki:Chugai Pharmaceutical Co., Ltd: Current Employment. Iwasaki:Chugai Pharmaceutical Co., Ltd.: Current Employment. Nogami:Chugai Pharmaceutical Co., Ltd, Novo Nordisk, Takeda, CSL, Sanofi, Bayer, Fujimoto Seiyaku, KM Bio, Sekisui Medical, Sysmex: Research Funding; Chugai Pharmaceutical Co., Ltd, Novo Nordisk, Takeda, CSL, Sanofi, Bayer, Fujimoto Seiyaku, KM Bio, Sekisui Medical, Sysmex: Honoraria.