Burixafor (GPC-100), a novel CXCR4 antagonist, has shown safe and effective mobilization of hematopoietic stem and progenitor cells (HSPC) in a phase I clinical study. In multiple myeloma patients, GPC-100 combined with granulocyte-colony stimulating factor (G-CSF) elicited a significant increase in circulating HSPCs that was comparable with the historical results from G-CSF plus the CXCR4 antagonist plerixafor. GPC-100 is currently being investigated for HSPC mobilization in combination with the FDA-approved ß-adrenergic receptor (ß-AR) blocker propranolol in a phase II clinical trial for autologous stem cell transplant (ASCT) in multiple myeloma. Here, we present preclinical findings indicating that the addition of propranolol to GPC-100 alone or GPC-100 and G-CSF combination can address the unmet needs of patients undergoing mobilization. Additionally, we show an enhanced and unique pattern of lymphoid cell mobilization that may have applications in adoptive cell therapies.
Compared to chemotherapy, ASCT significantly improves survival in multiple myeloma patients. Because HSPCs constitute a very small fraction (~ 0.1%) of circulating mononuclear cells, it is critical to mobilize a sufficient number of HSPC into peripheral blood during the ASCT process. Current therapies for HSPC mobilization include G-CSF as a monotherapy or combined with plerixafor. However, mobilization failure is experienced by 15-50% patients, leading to potential loss of ASCT as a treatment option and significant toxicity from repeated mobilization attempts. Moreover, G-CSF is contraindicated in sickle cell disease and may exacerbate autoimmune diseases. Therefore, our goal is to identify novel strategies that will not only address the mobilization failure, but also explore non-G-CSF options for adequate mobilization.
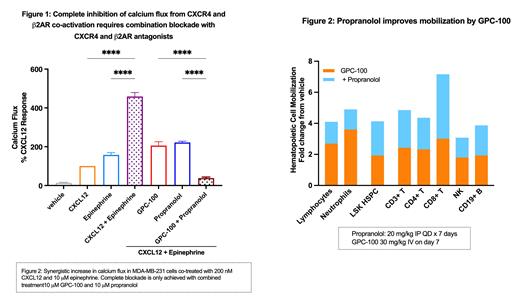
G protein-coupled receptors like CXCR4 can form heteromers with other receptors and result in altered signaling pathways. Using Proximity Ligation Assay in Namalwa (lymphoma) cells, we show that endogenously expressed CXCR4 and ß 2AR form heteromers, which are absent in CXCR4 knockout cells. In cell-based assays, co-activation of both receptors with their respective ligands, CXCL12 and epinephrine, leads to a synergistic increase in calcium flux (Figure 1) as well as ß-arrestin recruitment, indicating functional consequences of the heteromerization. Complete blockade of this enhanced signaling is only achieved with co-inhibition by both antagonists. Like CXCR4, ß 2AR is also expressed by HSPCs, and in lymph nodes was shown to heteromerize with CXCR4 to increase lymphocyte retention. In multiple myeloma patients, propranolol upregulated stem-cell like gene signature. Therefore, we sought to understand the effects of propranolol on HSPC mobilization by GPC-100.
In mice, single injection of GPC-100 effectively mobilized hematopoietic stem cells characterized as Lin negSca1 +cKit +(LSK) cells as well as mature Lin negCD34 + progenitor cells by 2-4-fold, respectively. Seven-day pretreatment with propranolol enhanced LSK cell mobilization by GPC-100 resulting in a 4-fold increase in circulating HSPCs (Figure 2). Addition of propranolol to G-CSF and GPC-100 led to 8-17-fold increases in mobilization of long-term repopulating HSCs (CD34 negLSK and CD150 +LSK), which were greater than the 4-7-fold increases elicited by G-CSF and G-CSF with plerixafor. Additionally, propranolol enhanced GPC-100 induced mobilization of white blood cells, neutrophils, and lymphocytes (Figure 2). Immune cell subset analyses revealed that GPC-100 induced a 3-fold increase in circulating CD8 +T cells, which was increased to 7-fold when combined with propranolol (Figure 2). For comparison, plerixafor increased CD8 + T cell mobilization by only 2-fold. GPC-100 induced mobilization of NK and B cells was also improved by propranolol.
These pre-clinical data support our phase II clinical trial currently in progress (NCT05561751). In the first arm of this two-arm trial, GPC-100 is combined with orally administered propranolol, and in the second arm, G-CSF is added to this combination. We anticipate that our clinical studies can fill the gap in the current standard of care without adding a significant financial burden or co-morbidities. Furthermore, enhanced mobilization of CD8+ T cells can provide extended opportunities for improved adoptive cell therapies.
Disclosures
Sukhtankar:GPCR Therapeutics: Current Employment. Cayton:GPCR Therapeutics: Current Employment. Zalicki:GPCR Therapeutics: Current Employment. Choi:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Mun:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Jo:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Kim:GPCR Therapeutics: Ended employment in the past 24 months. Lee:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Kim:GPCR Therapeutics: Ended employment in the past 24 months. Chin:GPCR therapeutics: Current Employment. Ramos:GPCR Therapeutics: Current Employment. Huh:GPCR Therapeutics: Current equity holder in private company. Kim:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Caculitan:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Jeong:GPCR Therapeutics: Current Employment. Cardarelli:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company.