Background
Thromboembolism (TE) is a complication in pediatric patients with congenital heart disease (CHD) due to disrupted blood flow, inflammation, foreign materials and cardiac surgery. Reported rates of TE in pediatric patients with CHD vary. Furthermore, most studies report TE rates following cardiac surgery, but there is a scarcity of data describing TE rates prior to cardiac surgery, the timing of these events, as well as the rates of post-operative TE recurrence in this population Given the limited information available describing the incidence and timing of TE both before and after cardiac surgery, the primary aim of this study was to describe the rate of TE 12 months before and 12 months after cardiac surgery in pediatric pts with CHD.
Methods
This was a single center retrospective study from October 2020 to June 2023 (inclusive). Patients were eligible for inclusion if they were <18 years of age, underwent cardiac surgery for congenital heart disease, and had a radiologic confirmed diagnosis of a thrombus either 12 months before or 12 months after CHD surgery. Data collection was conducted using the institution's surgical database and through electronic medical record chart review. Variables included age (neonates: <28 days of age, infants: 1 month to <12 months of age, children: 1 year to <12 years of age, and adolescents: 12 to 18 years of age), surgical procedure, administration of prothrombin complex concentrate, thrombotic history, cardiac catheterization ≤30 days of TE, lines at time of TE, treatment agents, and TE recurrence rates. Data analysis was conducted using descriptive statistics.
Results
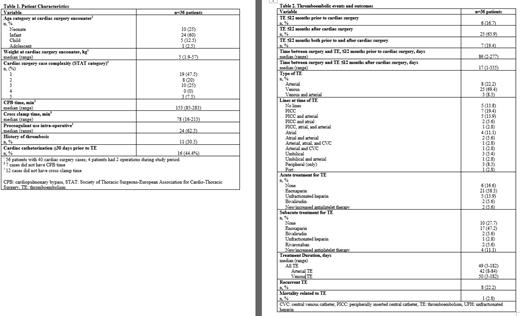
A total of 260 children underwent cardiac surgery during the study period, of these, 36 patients (14%) had a TE and were eligible for inclusion. Four patients had 2 operations during the study period for a total of 40 surgical encounters. The majority of patients were neonates and infants (85%) at the time of cardiac surgery (Table 1). Of the 36 (patients who had a TE, 6 (16.7%) patients had a TE prior to cardiac surgery, 23 (63.9%) had a TE after cardiac surgery, and 7 (19.4%) had a TE both before and after cardiac surgery. The median (range) time of TE occurring prior to or after cardiac surgery was 86 (2-277) days and 17 (1-335) days, respectively. Thromboembolism types included: 8 (22.2%) patients with arterial, 25 (69.4%) with venous, and 3 (8.3%) patients with both arterial and venous. Of note, 11 (20.5%) patients who developed thromboembolism had a history of prior TE >12 months prior to surgery. Twenty-four (62.5%) patients received prothrombin complex concentrate intra-op, and 16 (44.4%) patients had cardiac catheterization 30 days prior to the documented TE (Table 1). With regards to lines, 75% of TE were associated with a central venous/arterial catheter (Table 2). Treatment agents in the acute period included: no agent (6, 16.6%), unfractionated heparin (5, 13.9%), enoxaparin (21, 58.3%), bivalirudin (2, 5.6%), and new/increased antiplatelet therapy (2, 5.6%). Treatment agents in the subacute period included: no agent (10, 27.8%), enoxaparin (17, 47.2%), bivalirudin (2, 5.6%), unfractionated heparin (1, 2.8%), rivaroxaban (2, 5.6%), and new/increased antiplatelet therapy (4, 11.1%%). Overall median (range) duration of treatment was 49 (3-182) days. Of the 36 patients, 8 (21.2%) patients had at least 1 recurrent thromboembolism (Table 2).
Conclusions
We report in this single center retrospective study a high incidence of TE in children with CHD not only after cardiac surgery but also prior to surgery. Awareness of TE prior to cardiac surgery may be beneficial for optimizing post-operative prevention strategies to minimize the recurrence of TE. Future multicenter prospective studies are needed to validate these findings and evaluate risks factors for TE in pediatric patients with CHD both before and after cardiac surgery.
Disclosures
Betensky:Janssen Pharmaceuticals: Consultancy, Honoraria. Goldenberg:Boehringer-Ingelheim: Consultancy; Astra Zeneca: Consultancy; Bayer: Consultancy; Daiichi Sankyo: Consultancy; Chiesi: Consultancy; University of Colorado-affiliated Academic Research Organization CPC Clinical Research: Other: Serves on clinical trials oversite committees for pharma studies; Novartis: Other: Data and Safety Monitoring Committee; Anthos Therapeutics: Consultancy.