Introduction: Brexu-cel is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the United States for the treatment of adults with R/R MCL and in the European Union for adults with R/R MCL who received ≥2 prior therapies, including a Bruton tyrosine kinase inhibitor (BTKi). After a median of 35.6-months follow-up in the pivotal Phase 2, multicenter ZUMA-2 study, brexu-cel demonstrated an objective response rate (ORR) of 91%, a complete response (CR) rate of 68%, and a median overall survival (OS) of 46.6 months in all 68 treated patients with R/R MCL and a median OS not reached in patients with a CR (Wang et al. J Clin Oncol. 2022). ZUMA-18 is a multicenter, open-label, expanded access study of brexu-cel for the treatment of patients with R/R MCL. Here, we report OS outcomes after 4 years in ZUMA-2 and the primary analysis of ZUMA-18.
Methods: In ZUMA-2, adults (≥18 years) with R/R MCL with ≤5 prior regimens including a BTKi underwent leukapheresis and conditioning chemotherapy followed by one infusion of brexu-cel (2×10 6 anti-CD19 CAR T cells/kg). ZUMA-18 consisted of 2 cohorts. The primary objectives were to provide access to brexu-cel for patients with R/R MCL until it was commercially available (Cohort 1) and patients with R/R MCL whose manufactured product did not meet commercial release specifications (Cohort 2). In Cohort 1, adults (≥18 years) with R/R MCL with ≥1 prior regimen underwent leukapheresis and conditioning chemotherapy followed by a single infusion of brexu-cel at a target dose of 2×10 6 cells/kg (or fixed dose of 2x10 8 anti-CD19 CAR T cells for patients who are ≥100 kg). In Cohort 2, patients received Cohort 1 treatment without leukapheresis (initial leukapheresis product used). Key endpoints were safety, ORR, and OS.
Results: As of July 23, 2022, median follow-up in all 68 treated patients in ZUMA-2 was 47.5 months (range, 37.9-68.3) and median OS was 46.4 months (range, 1.2-63.0) with 30 patients (44%) still alive. Median (range) OS for patients with CR (n=46), partial response (PR; n=16), and no response (n=6) were 58.7 (4.8-61.9), 16.3 (1.2-63.0), and 8.5 months (2.3-22.9), respectively.
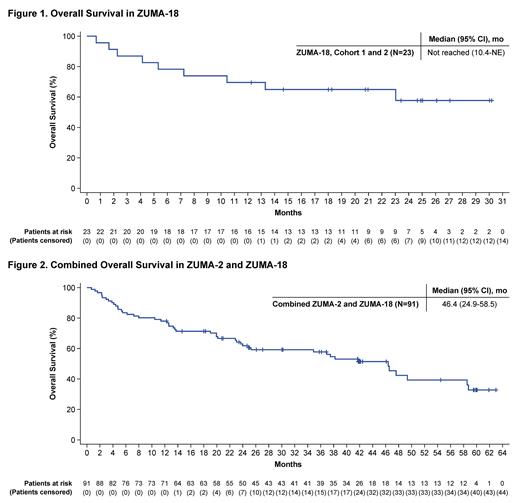
As of February 3, 2023, 27 patients were enrolled in ZUMA-18 and 23 (Cohort 1, n=21; Cohort 2, n=2) received brexu-cel with a median follow-up of 33.5 months (range, 24.5-35.3). Median age was 69.0 years (range, 43-79); 78% were male; and median number of prior regimens was 4 (range, 1-10). The investigator-assessed ORR was 87% (95% CI, 66.4-97.2); 13 patients (57%), including both patients in Cohort 2, had a CR (95% CI, 34.5-76.8), 7 patients (30%) had a PR (95% CI, 13.2-52.9), and 2 patients (9%) had progressive disease (PD) as their best response to brexu-cel (95% CI, 1.1-28.0; 1 patient had not been assessed at data cutoff). The median OS was not reached (95% CI, 10.4-not estimable) at data cutoff with a 58% OS rate at 24 months (Figure 1). At data cutoff, 14 patients (61%) were still alive and 9 patients (39%) had died; 5 due to adverse events (AEs), 2 due to PD, and 2 due to other causes.
All 23 patients in ZUMA-18 experienced at least 1 Grade ≥3 AE and 18 patients (78%) experienced at least 1 Grade ≥3 treatment-related AE, of which, neutropenia (43%), anemia (30%), thrombocytopenia (30%), encephalopathy (22%), and leukopenia (22%) were most common. Grade ≥3 cytokine release syndrome and neurological events occurred in 1 patient (4%) and 8 patients (35%), respectively. Five Grade 5 AEs occurred, 1 that was deemed related to brexu-cel therapy (multiple organ dysfunction syndrome on Day 20) and 4 that were deemed unrelated to brexu-cel therapy (sepsis [2, Days 123 and 219], aspiration [1, on Day 49], and encephalopathy [1, on Day 68]).
The combined median OS for ZUMA-2 and ZUMA-18 patients (N=91) was 46.4 months (range, 0.7-63.0) with a 62% OS rate at 24 months (Figure 2).
Conclusions: With 4 years of median follow-up in ZUMA-2, patients continued to benefit from brexu-cel with a median OS of almost 5 years in patients with CR. Consistent with ZUMA-2 findings, brexu-cel demonstrated a high level of efficacy in patients with R/R MCL in ZUMA-18, with an ORR of 87% and median OS not yet reached at 33.5 months of follow-up. Additionally, no new safety signals were detected in ZUMA-18. Of note, given the small sample size (n=2), no definitive conclusions can be drawn from Cohort 2 outcomes alone. Together, these results support the continued use of brexu-cel in the R/R MCL setting.
Disclosures
Goy:Resilience: Current holder of stock options in a privately-held company; OncLive Peer Exchange: Honoraria; Practice Update Oncology: Consultancy, Honoraria; AbbVie/ Pharmacyclics LLC: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; Acerta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics LLC, an AbbVie Company: Other: Steering Committee, Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Karyopharm: Research Funding; Infinity: Research Funding; Seagen: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Clinical Advances in Hematology & Oncology: Consultancy; Novartis: Consultancy, Honoraria; Genentech: Research Funding; Michael J. Hennessey: Consultancy, Honoraria; Medscape: Consultancy; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; Regional Cancer Care Associates, OMI: Current Employment, Research Funding; Verastem: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; Physicians Education Resource, LLC: Consultancy, Honoraria, Other: travel, accommodations, and expenses; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other; MorphoSys: Research Funding; Alloplex: Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genomics Testing Cooperative LLC: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership Role; COTA Healthcare: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership Role; OMI: Current Employment; Constellation: Research Funding; Hoffman la Roche: Consultancy, Honoraria, Research Funding; Xcenda: Consultancy, Honoraria. Jacobson:Novartis: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Abbvie: Consultancy; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy; Abintus Bio: Consultancy; Caribou Bio: Consultancy; Instil Bio: Consultancy; ImmPACT Bio: Consultancy; Daiichi-Sankyo: Consultancy; Ipsen: Consultancy; Morphosys: Consultancy; Synthekine: Consultancy; Pfizer: Research Funding; Miltenyi Biotec: Consultancy; Kite, a Gilead company: Consultancy, Research Funding. Flinn:Portola Pharmaceuticals: Research Funding; Nurix: Research Funding; Millennium Pharmaceuticals: Research Funding; Marker Therapeutics: Research Funding; Loxo: Research Funding; Infinity Pharmaceuticals: Research Funding; Incyte: Research Funding; IGM Biosciences: Research Funding; Forty Seven: Research Funding; Forma Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Epizyme: Research Funding; CTI Biopharma: Research Funding; Curis: Research Funding; Constellation Pharmaceuticals: Research Funding; City of Hope National Medical Center: Research Funding; Celgene: Research Funding; CALGB: Research Funding; CALIBR: Research Funding; Bristol Myers Squibb: Research Funding; Biopath: Research Funding; ArQule: Research Funding; Agios: Research Funding; 2seventy bio: Research Funding; Xencor: Consultancy; Vincerx Pharma: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Servier Pharmaceuticals: Consultancy; Secura Bio: Consultancy; Novartis: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Genmab: Consultancy; AbbVie: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Seagen: Research Funding; MorphoSys: Research Funding; Merck: Research Funding; Karyopharm: Research Funding; Juno: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Iksuda: Consultancy; Hutchison MediPharma: Consultancy; Great Point Partners: Consultancy; Gilead Sciences: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Century Therapeutics: Consultancy; BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Acerta Pharma: Consultancy, Research Funding; Rhizen Pharmaceuticals: Research Funding; Roche: Research Funding; Step Pharma: Research Funding; Tessa Therapeutics: Research Funding; Trillium Therapeutics: Research Funding; BeiGene: Consultancy; Seattle Genetics: Research Funding; Triphase Research & Development Corp.: Research Funding; Unum Therapeutics: Research Funding. Hill:Incyte: Consultancy; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Genentech: Consultancy, Other: Advisory board, Research Funding; Bristol Myers Squibb: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding; Gilead: Other: Advisory board. Olalekan:Gilead Sciences: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy; ADC: Consultancy; Curio Science: Consultancy; Epizyme: Consultancy; Janssen: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Nektar: Consultancy; Novartis: Consultancy; Syncopation: Consultancy; TG Therapeutics: Consultancy; Allogene: Research Funding; Daiichi Sankyo: Research Funding. Zheng:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Honoraria, Other: travel support. Nunes:Amgen: Current holder of stock options in a privately-held company; Gilead Sciences Europe Ltd: Current Employment, Current holder of stock options in a privately-held company, Honoraria. Zhang:Kite, a Gilead Company: Current Employment. Shen:Atara: Other: Intellectual property , Patents & Royalties; Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: Intellectual property , Patents & Royalties. Kloos:Kite, a Gilead Company: Current Employment. Wang:Mumbai Hematology Group: Honoraria; OMI: Honoraria; Pharmacyclics: Honoraria; Physicians Education Resources: Honoraria; Genentech: Consultancy, Research Funding; DTRM Biopharma (Cayman) Limited: Consultancy; Deciphera: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Be Biopharma: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; InnoCare: Consultancy, Research Funding; Epizyme: Consultancy, Honoraria; Hebei Cancer Prevention Federation: Honoraria; Juno Therapeutics: Research Funding; Studio ER Congressi: Honoraria; Celgene: Other: Travel, Research Funding; Scripps: Honoraria; Vincerx: Research Funding; Loxo Oncology: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Anticancer Association: Honoraria; Practice Point Communications: Honoraria; TS Oncology: Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; Milken Institute: Consultancy; Oncternal: Consultancy, Research Funding; Parexel: Consultancy; OncLive: Honoraria; Molecular Templates: Research Funding; Imedex: Honoraria; BGICS: Honoraria; Clinical Care Options: Honoraria; Pepromene Bio: Consultancy; Pharmacyclics: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Practice Point Communications (PPC): Honoraria; CSTone: Consultancy; WebMD: Honoraria; Genentech: Consultancy, Research Funding; NIH: Honoraria; Moffit Cancer Center: Honoraria; Nurix: Honoraria; Oncology Specialty Group: Honoraria; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MJH Life Sciences: Honoraria; MD Education: Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; IDEOlogy Health: Honoraria; i3Health: Honoraria; Genmab: Honoraria, Research Funding; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria, Other: Travel; CAHON: Honoraria; Bantam Pharmaceutical: Honoraria.