Abstract
As most patients with sickle cell disease (SCD) do not have access to curative therapies, the availability of drug therapies that can modify disease severity remains highly desirable. Despite an increased understanding of the pathophysiology of SCD, only 4 drugs are approved by the US Food and Drugs Administration. Most drug trials in SCD have involved the use of acute pain episodes as the primary clinical end point. These studies have typically been to prevent or shorten the duration of such episodes. To date, no drug has received regulatory approval for shortening the duration of acute vaso-occlusive complications, likely highlighting the complex pathophysiology of acute pain episodes. Trials to prevent acute pain episodes have largely evaluated those episodes requiring health care use as a surrogate end point. However, with differences in culture and health care practices among countries, health care use may not reliably predict clinically important effects on acute pain episodes. This article discusses issues related to the use of health care use as the primary end point for prevention trials of acute pain episodes and highlights the importance of evaluating patient-reported outcomes as well as other SCD-related complications as outcome measures.
Introduction
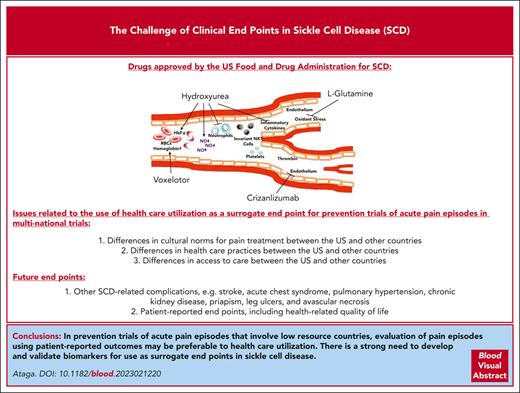
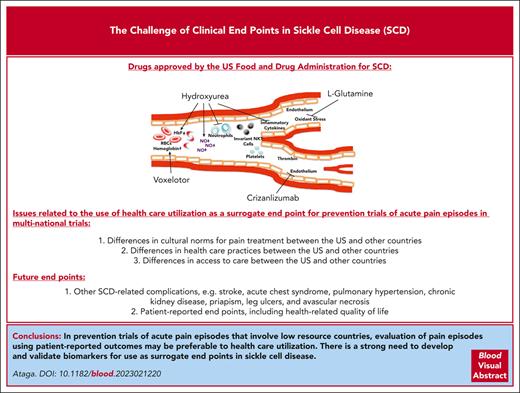
Sickle cell disease (SCD), including homozygous sickle cell disease (sickle hemoglobin S [HbSS]), compound heterozygous forms such as sickle hemoglobin C disease (HbSC), and sickle beta thalassemia (HbSβ0 and HbSβ+), affects millions of individuals worldwide.1 SCD is particularly prevalent in sub-Saharan Africa, where an estimated 230 000 children with sickle cell anemia (a term now commonly used to refer to HbSS and HbSβ0) were born in 2010, representing up to 75% of worldwide births.2 SCD is characterized by the presence of sickle hemoglobin, chronic hemolytic anemia, vaso-occlusive complications, and progressive end-organ damage. Although most children with SCD in resource-rich countries now live to adulthood,3-5 the mortality rate remains high in sub-Saharan Africa for children with sickle cell anemia, with estimated mortality rates of 36.4% (range, 33.4%-39.4%) for those younger than 5 years and 43.3% (range, 39.3%-47.3%) for those aged younger than 10 years.6 Despite the improved survival of children in resource-rich nations, the life expectancy of patients with sickle cell anemia remains ∼3 decades shorter than that of the general population.7-10 Although SCD may be cured after allogeneic stem cell transplantation and possibly after gene therapy and gene editing,11-13 these curative modalities remain unavailable to most patients. This makes the availability and access to safe, effective, and affordable drugs highly desirable. Four drugs, hydroxyurea, l-glutamine, crizanlizumab, and voxelotor, are currently approved by the US Food and Drug Administration (FDA) specifically for SCD, but trials of multiple other promising drugs have been unsuccessful. While this is, in large part, due to a lack of clinical efficacy of the tested drugs, there is some concern that the negative results in some clinical trials may have been due to the choice of the primary clinical end point.
This article discusses clinical end points for drug trials in SCD, highlighting the focus on acute pain episodes and the challenge of selecting appropriate clinical end points.
Pathophysiology
An adequate understanding of the pathophysiology of SCD is necessary for the development of effective drug therapies for SCD. The pathophysiology of SCD is primarily because of the polymerization of HbS when it is deoxygenated.14 The rate and extent of polymer formation depends on the degree and duration of HbS deoxygenation, the concentration of HbS in the red blood cell (RBC), and the amount of fetal hemoglobin (HbF).14 Polymerization of HbS as well as its multiple consequences, such as increased oxidant stress, inflammation, endothelial cell injury, endothelial dysfunction, complement activation, platelet activation, and coagulation activation, may be targets in the development of drug therapies for SCD. Two major pathophysiological processes, hemolysis and vaso-occlusion, are thought to drive the clinical manifestations of SCD.1 A division of SCD into 2 overlapping clinical subphenotypes has been suggested: viscosity–vaso-occlusion (in which patients have higher hemoglobin levels, possibly increased blood viscosity, and complications such as avascular necrosis of bone, acute pain episodes, and acute chest syndrome) and hemolysis–endothelial dysfunction (in which patients have increased anemia and higher levels of measures of hemolysis and complications such as stroke, pulmonary hypertension, priapism, and leg ulcers).15 Although simplistic and somewhat controversial, this description may provide more insight into the pathobiology of SCD-related complications and the effects of therapies. The pathophysiology of SCD is reviewed more extensively elsewhere.16
Historical drug trials for acute pain episodes in SCD
The choice of outcome measures is one of the most important considerations in designing clinical trials.17 Most drug trials in SCD over the past 30 years have used acute pain episodes (commonly referred to as vaso-occlusive crises or events) as the primary clinical end point.18 This is understandable because acute pain episodes represent the most frequent reason for medical contact by patients. The current approaches to the management of acute pain episodes are prevention, support, and intervention. Despite multiple attempts, no drugs have, to date, received regulatory approval for shortening the duration of acute pain episodes (Table 1). There are no validated tests or biomarkers that confirm the absence or resolution of acute pain episodes. Consequently, clinical trials of ongoing pain episodes have used end points that suggest resolution or substantial improvement of such episodes. Multiple large clinical trials of initially promising drugs that were evaluated during acute vaso-occlusive episodes are worth highlighting. Poloxamer-188, a nonionic block copolymer surfactant with hemorheologic and antithrombotic properties, was evaluated in two phase 3 trials in children and adults hospitalized for acute pain episodes. In the earlier study, treatment with poloxamer-188 significantly, albeit modestly, shortened the duration of acute pain episodes (time of randomization to time of protocol-defined crisis resolution) when compared with placebo (141 h vs 133 h; difference, 9 h; P = .04). However, there were concerns regarding the validity of the results due to an absence of documentation of the study’s stringent crisis resolution criteria in 30% of participants in the placebo arm and in 18% of participants in the poloxamer-188 arm.21 With a verified primary end point (time to discontinuation of IV opioids) in 99% of all participants, a subsequent phase 3 trial showed no significant benefit of poloxamer-188 compared with placebo (81.8 hours vs 77.8 hours; P = .09).22 Rivipansel (GMI-1070), a small-molecule pan-selectin inhibitor that binds predominantly to E-selectin, reduced the cumulative IV opioid dose utilized during an acute pain episode by 83% compared with a placebo in a phase 2 study.29 However, a phase 3 trial did not show significant benefit for rivipansel in shortening the duration of hospitalization (time to readiness-for-discharge or time to discharge) or IV opioid use (time to discontinuation of IV opioids).30 Although studies of inhaled nitric oxide did not show benefit for acute pain episodes or acute chest syndrome,23,24 the nitric oxide precursor, l-arginine, significantly reduced total analgesia, pain scores, time to crisis resolution, and the total length of hospital stay.26,27 The outcome measures most commonly used to assess resolution of acute pain episodes, that is, the time to discontinuation of IV opioids and time to readiness-for-discharge, are thought to be clinically meaningful end points in this setting. The lack of benefit for acute pain episodes observed in most drug trials may reflect the complex pathophysiology of pain in SCD. However, the reported benefits with l-arginine are encouraging, and further studies are required to confirm these findings.
Most studies of acute pain episodes have focused on the use of drug therapies to prevent this complication (Table 2). These studies have often evaluated those pain episodes requiring health care utilization (ie, emergency department visits, infusion or day hospital visits, hospitalizations, or other physician contact) during which time the patient is treated with a parenteral or oral analgesic as a surrogate end point. As most pain episodes are treated at home,46-48 these episodes that substantially contribute to disease morbidity and decreased quality of life are increasingly being evaluated as key or secondary end points. However, assessing what constitutes an acute pain episode still remains a challenge. Should these be restricted to only those episodes severe enough to require opioid analgesics or should they be based on a defined severity on a numeric pain scale? This issue is confounded by the fact that use of opioid analgesics may be influenced by multiple factors, including but not limited to access to and availability of medication, pain tolerance, and past experiences. Three drugs, hydroxyurea, l-glutamine, and crizanlizumab, are currently approved by the US FDA for SCD based on reduction in the frequency of acute pain episodes. Randomized phase 3 clinical trials in adults and infants with sickle cell anemia showed that treatment with hydroxyurea significantly reduced acute pain episodes, acute chest syndrome, transfusion requirements, and hospitalizations.36,37 Treatment with l-glutamine, a conditionally essential amino acid (of which increased levels are needed in situations such as stress), significantly reduced the median number of acute pain episodes (3.0 vs 4.0; P = .005), hospitalizations (2.0 vs 3.0; P = .005), cumulative hospital days, and the frequency of acute chest syndrome compared with placebo.41 Crizanlizumab is a humanized monoclonal anti–P-selectin antibody that blocks the interaction between P-selectin (expressed on endothelial cells and platelets) and its ligand, P-selectin glycoprotein ligand 1. In a randomized, placebo-controlled phase 2 trial, treatment with high-dose crizanlizumab resulted in a significantly lower rate of acute pain episodes (1.63 vs 2.98; P = .01) and increased the median times to first (4.07 months vs 1.38 months; P = .001) and second pain episodes (10.32 vs 5.09 months; P = .02) than that of placebo.42
Multiple other promising drugs that have been evaluated for the prevention of acute pain episodes have reported no significant benefit. Senicapoc, which selectively blocks the calcium-activated potassium efflux (Gardos) channel, improves anemia and hemolysis in SCD.39,49 However, despite these improvements, a phase 3 trial showed no significant decrease in the rate of acute pain episodes compared with placebo.39 Based on the data that ticlopidine, a P2Y12 adenosine 5′-diphosphate–receptor antagonist, decreased the number of acute pain episodes, mean duration of acute pain episodes, and severity of such episodes, studies of 2 new generation P2Y12 receptor blockers were recently conducted. In a phase 3, placebo-controlled study of children with sickle cell anemia, prasugrel did not significantly lower the rate of vaso-occlusive events compared with placebo.40 In the phase 3 HESTIA3 study, no reduction in the yearly incidence of vaso-occlusive episodes (a composite of acute pain episodes and/or acute chest syndrome) was seen with ticagrelor vs placebo.45 Although tovinontrine (IMR-687), an oral selective phosphodiesterase inhibitor in RBCs that increases cyclic guanosine monophosphate levels to reactivate HbF, was generally well tolerated, it did not statistically decrease the annualized rate of vaso-occlusive episodes compared with placebo.50 In a randomized, double-blind, multicenter phase 2a study of children and young adults with sickle cell anemia and elevated high-sensitivity C-reactive protein >1.0 mg/L at screening, treatment with canakinumab, an interleukin-1β neutralizing antibody, for 6 months did not significantly reduce average daily pain.44 Encouragingly, however, treatment with canakinumab reduced the levels of multiple biomarkers of inflammation as well as the number and duration of hospitalizations, with trends for improvement in pain intensity, fatigue, and absences from school or work.
Despite the approval of crizanlizumab for reduction of acute pain episodes in adults and children aged ≥16 years, a recently completed multinational phase 3 trial (STAND) surprisingly showed no significant difference between crizanlizumab 5 mg/kg every 4 weeks (the approved dose) or crizanlizumab 7.5 mg/kg every 4 weeks vs placebo in reducing the annualized rates of vaso-occlusive episodes leading to a health care visit over the first year after randomization.51 Based on the robust benefits demonstrated in the earlier phase 2 SUSTAIN trial,42 combined with reports in other patient populations with SCD,52-54 this result was unexpected. Although the reason(s) for the negative result of the STAND trial remains uncertain, it highlights the concerns regarding the use of health care utilization as a surrogate for acute pain episodes, particularly in studies performed in multiple countries. With differences in cultural norms for pain treatment, access to care, and health care practices and use among the US and countries in Europe, the Middle East, South America, and sub-Saharan Africa,55-57 it is easy to see how this surrogate end point could be problematic in multicountry clinical trials. In environments with low health care use, the assessment of this surrogate end point is unlikely to reliably predict achievement of clinically important effects on acute pain episodes. It is not an exaggeration to state that most historical studies of vaso-occlusive episodes have not truly evaluated acute pain episodes but rather health care use for such episodes. Genetic heterogeneity and environmental conditions in different countries may also contribute to negative trial results in SCD.
Other clinical end points for SCD trials
With the myriad of complications experienced by patients with SCD, there is increasing recognition of the importance of studying other clinical events including acute chest syndrome, ischemic stroke, chronic kidney disease, priapism, pulmonary hypertension, leg ulcers, and avascular necrosis of large bones. Fatigue is also a significant concern for children and adults with SCD.58 Rather than suggest new clinical end points, this article seeks to highlight the recommendations of a panel of clinicians, investigators, and patients engaged by the American Society of Hematology and the US FDA.59,60 Indeed, clinical trial end points for SCD based on brain, kidney, and cardiovascular complications as well as patient-reported outcomes have been published.59,60 Hydroxyurea has been evaluated for primary and secondary prevention of stroke. Hydroxyurea was noninferior to chronic RBC transfusion in preventing stroke in individuals with abnormal transcranial Doppler (TCD) velocities (≥200 cm/s) who had been on monthly blood transfusion for at least 12 months and had no severe vasculopathy.61 In a double-blind, parallel-group trial of children with sickle cell anemia and abnormal TCD velocities conducted in Nigeria, no significant difference was seen in the stroke incidence rate with low-dose hydroxyurea (10 mg/kg) compared with moderate-dose hydroxyurea (20 mg/kg).62 A phase 3 trial to evaluate the effect of voxelotor on conditional TCD velocity (≥170 to <200 cm/s) in children with sickle cell anemia is ongoing (NCT04218084). Although the multicenter, phase 3 Stroke With Transfusions Changing to Hydroxyurea study comparing the effects of RBC transfusions and iron chelation vs hydroxyurea and phlebotomy on the composite primary end point of stroke and iron overload was stopped for futility (due to equivalent liver iron content in both treatment groups),63 no significant difference in the incidence rates of the primary outcome measures of stroke, transient ischemic attack, and death was seen in another study that compared low-dose (10 mg/kg daily) vs moderate-dose (20 mg/kg daily) hydroxyurea for secondary prevention of stroke.64
Because some SCD-related complications occur sporadically and over long periods, evaluation of these end points may require enrollment of large numbers of participants and/or long study duration to observe and quantify any treatment effects. As such, the presence of suitable surrogate end points may facilitate the conduct of short-term studies to evaluate the efficacy of therapies in these clinical settings and support the design and planning of longer-term studies required for regulatory approval.65 Multiple clinical and laboratory biomarkers, defined as characteristics that are objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention,66 are described in SCD. Although an increased TCD velocity of at least 200 cm/s is associated with an increased risk of stroke in children with sickle cell anemia and has been validated as an acceptable surrogate end point,67,68 the majority of biomarkers require validation of their prognostic importance before they are acceptable as suitable surrogate end points. Based on the results of the HOPE trial,69 hemoglobin concentration has recently been accepted as a suitable surrogate end point for accelerated drug approval in SCD, contingent “upon verification and description of clinical benefit in confirmatory trial(s).” With this approval of voxelotor based on increase in hemoglobin, reanalysis of data from the phase 3 trial of senicapoc showed a hemoglobin response >1g/dL from baseline in 29 of 85 patients (34%) in the senicapoc arm vs 6% in the placebo group treated for at least 24 weeks,70 similar to the findings in patients who received 900 mg per day of voxelotor in the HOPE trial but with more pronounced improvement of markers of hemolysis. In view of these data, it is conceivable that by using increased hemoglobin as the primary end point, the phase 3 senicapoc trial may have been considered a success even in the absence of reduction in the frequency of acute pain episodes. Although no drugs have, as yet, been approved based on HbF as a surrogate end point, with the plethora of epidemiological and clinical data demonstrating its disease-mitigating effects in SCD,7,71,72 increase in HbF may be a suitable surrogate end point for drug development. However, a clinically meaningful increase in HbF would need to be adequately defined.
Patient-reported outcomes (PROs), defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else,”73 can complement traditional efficacy outcomes. Because pain and fatigue, the most common symptoms in SCD, are subjective, the use of PROs as efficacy end points is particularly important.60 Health-related quality of life (HRQoL), a type of PRO, is a suitable end point for drug development.74 Substantial impairment of HRQoL is present among patients with SCD75 and with complications such as acute pain episodes, acute chest syndrome, and stroke.76,77 Favorable response to hydroxyurea is associated with improved HRQoL in children78 and adults.79 Instruments, including the Patient-Reported Outcomes Measurement Information System,80 the closely related Adult Sickle Cell Quality of Life Measurement Information System, and the Pediatric Quality of Life Inventory, which contains both general and SCD-specific modules of PROs for children, are increasingly being used to evaluate the effect of drug therapies.
Conclusions
Although acute pain episodes are the most common clinical end points for drug trials in SCD, the difficulty in adequately defining such episodes may contribute to negative trial results. Health care use, particularly in trials involving high and low resource countries, may not be an ideal surrogate end point for acute pain episodes. In the absence of more reliable outcome measures or validated surrogates, evaluation of acute pain episodes regardless of health care use, that is, including episodes at home, may be a preferred approach to assess vaso-occlusive episodes in trials across different countries. With multiple complications associated with SCD, it is important that other clinical end points, including PROs and SCD-related complications, are considered in the assessment of drug therapies. The pathophysiology of SCD and its specific complications must be considered in the choice of clinical end points. As demonstrated by the successful use of TCD velocity in trials of primary stroke prevention, there is a strong need to develop and validate more biomarkers for use as surrogate end points, especially for complications that occur sporadically and over long periods; however, confirmatory studies demonstrating clinical benefit and safety will likely be required.
Acknowledgments
K.I.A. is supported by awards from the US Food and Drug Administration (FD006030) and National Institutes of Health, National Heart, Lung and Blood Institute (HL159376).
Authorship
Contribution K.I.A. wrote the paper.
Conflict-of-interest disclosure: K.I.A. has received research funding from Novartis, Novo Nordisk, and Takeda Pharmaceuticals; served on advisory boards for Novartis, Novo Nordisk, Agios Pharmaceuticals, Fulcrum Therapeutics, and Pfizer; served as a consultant for Roche and Biomarin; and serves on a data-monitoring committee for Vertex.
Correspondence: Kenneth I. Ataga, Center for Sickle Cell Disease, University of Tennessee Health Science Center at Memphis, 956 Court Ave, Suite D324, Memphis, TN 38163; e-mail: kataga@uthsc.edu.