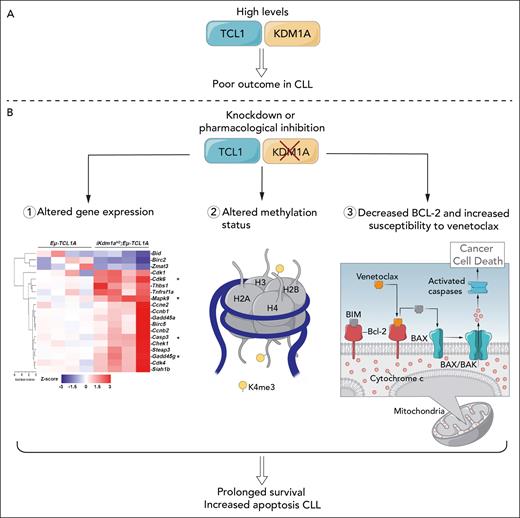
In this issue of Blood,1Jiang et al demonstrate that KDM1A is upregulated in malignant B cells and that this is associated with aggressive disease as well as adverse outcome in samples from the CLL8 clinical trial2 (see figure panel A). They further show that knockdown of Kdm1a in a murine model altered the epigenome.
(A) KDM1A interacts with TCL1 and is increased in CLL. Higher levels are associated with more aggressive disease and shorter response to chemoimmunotherapy. (B) Knockdown of KDM1A in a mouse model alters gene expression, notably of genes regulating apoptosis and motility, alters methylation status, and works synergistically with agents, such as venetoclax, to increase CLL cell killing. BAK, BCL2 antagonist/killer 1; BAX, BCL2 associated X; BCL2, B-cell lymphoma 2; BIM, Bcl-2 interacting mediator of cell death. Professional illustration by Patrick Lane, ScEYEnce Studios.
(A) KDM1A interacts with TCL1 and is increased in CLL. Higher levels are associated with more aggressive disease and shorter response to chemoimmunotherapy. (B) Knockdown of KDM1A in a mouse model alters gene expression, notably of genes regulating apoptosis and motility, alters methylation status, and works synergistically with agents, such as venetoclax, to increase CLL cell killing. BAK, BCL2 antagonist/killer 1; BAX, BCL2 associated X; BCL2, B-cell lymphoma 2; BIM, Bcl-2 interacting mediator of cell death. Professional illustration by Patrick Lane, ScEYEnce Studios.
Epigenetic changes are known to drive clonal evolution and diversity in chronic lymphocytic leukemia (CLL), making CLL a good model to study epigenetic evolution over time.3,4 However, the molecular mechanisms that drive these changes are poorly understood. To understand the mechanisms better, the authors generated a doxorubicin (Dox)-inducible Kdm1a knockdown in Eμ-TCL1 mice (Kdm1a-KD). Kdm1a-KD mice had reduced tumor burden, prolonged survival, upregulation of p53 and proapoptotic pathways, and changes in the tumor microenvironment (TME), indicating an important role for KDMIA. So, how does this occur and what, if anything, does this have to do with the CLL epigenome? In elegant and comprehensive experiments, the authors analyzed differences in global transcriptome by RNA sequencing as well as H3K4me3 marks by chromatin immunoprecipitation sequencing between Eμ-TCL1 and Kdm1a-KD mice and then confirmed their findings in human CLL samples. Their findings demonstrate that in CLL cells, KDM1A alters histone methylation patterns in pathways regulating cell death and motility, thereby acting as an oncogenic transcriptional repressor in this disease. More important, they were able to demonstrate this not only in their murine model, but also in primary human CLL cells. Last, they demonstrate marked synergisms of pharmacologic KDM1A inhibition with other currently available agents, which they suggest provides a strong rationale to investigate targeting of KDM1A in CLL (see figure panel B).
It is always important to understand if the murine model being used is a good model for the question being asked. Eμ-TCL1 transgenic mice are an established, well-characterized model of aggressive, unmutated immunoglobulin heavy chain variable CLL.5 The changes in the TME closely resemble those seen in human disease.6,7 This mouse model also recapitulates changes in the methylome in CLL.8 The authors’ starting point was to study the interactome of T-cell leukemia/lymphoma 1 (TCL1), so it is of course logical to start with this TCL1 transgenic mouse model. In their studies, they identified 1000 TCL1-interacting proteins, some of which were chromatin-modifying enzymes. For several reasons outlined in the article, they focused attention on KDM1A and verified the expected interaction with TCL1. On the basis of the observation that increased TCL1A expression led to increased histone methylation activity, they postulated that increased TCL1A expression in CLL cells affected the epigenetic signature by modulating KDM1A-mediated demethylase activity. The findings are novel as there are no previous reports on the role of KDM1A in CLL, and they were able to confirm their findings by demonstrating higher KDM1A levels of expression in CLL and other leukemia cells compared with healthy B cells and that levels of expression increased with disease progression. Their studies in the Dox-induced KDM1A knockdown model demonstrated the potential importance of this pathway as the Kdm1a-KD mice had longer survival and slower disease progression. Changes in the T-cell and TME environment in Eμ-TCL1 mice have been shown to closely mimic that seen in human disease.6 In both Eμ-TCL1 mice and in human cell lines in which KDM1A expression was knocked down, the observed results suggest a direct effect not only on CLL cells but also on the interaction of CLL cells with various components of the TME. Their studies further suggest that KDM1A expression regulates global transcriptional activity in CLL and that KDM1A knockdown led to differential H3K4me3 enrichment occupancy in areas regulating genes involved in apoptosis pathways and cell migration/adhesion. Their analyses from the CLL8 trial patient samples show that KDM1A is associated with more aggressive disease and poorer clinical outcome.
But why is any of this work of importance to us in thinking about treating CLL, a disease in which recent advances in treatment have revolutionized outcomes and in which there is no longer much relevance for the use of chemoimmunotherapy?9 As shown in figure panel B, when the authors employed a variety of KDM1A inhibitors, they found that at least some of these, notably C12, had not only the expected activity in terms of changes in apoptotic cell death in CLL cells, H3K9me3 levels, and poly (ADP-ribose) polymerase cleavage, but also, and of more potential interest, had synergistic activity with agents, such as venetoclax, which is one of the mainstays of our current targeted treatment approaches in CLL. KDM1A inhibitors are already being explored in other malignancies, both alone and in combination.10 Obviously, much work needs to be done to characterize potential KDM1A inhibitors, including their safety profile in early-phase clinical trials, and that the selected inhibitors recapitulate the synergistic activity with agents such as venetoclax.
In conclusion, I agree fully with the authors’ conclusions that they have provided sufficient evidence to support that KTM1A appears to be a target worthy of further investigation in CLL.
Conflict-of-interest disclosure: J.G.G. receives honoraria from AbbVie, Amgen, AZ BMS, Kite/Gilead, and Janssen.