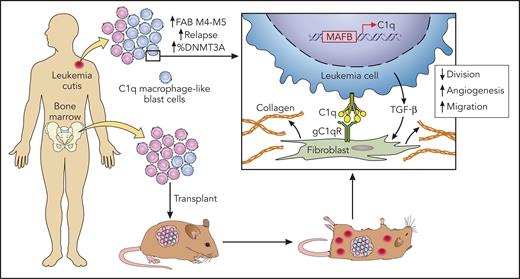
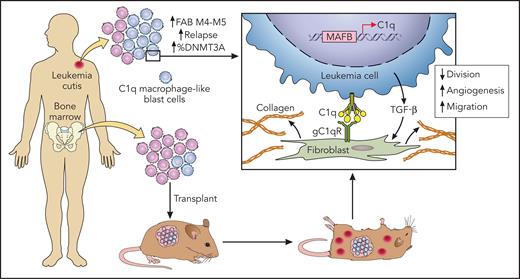
In this issue of Blood, Yang et al1 identified that the complement component 1q (C1q) may facilitate both the dissemination of acute myeloid leukemia (AML) outside the bone marrow and relapse.
The common idea that AML is a homogeneous disease of the bone marrow is challenged by the occurrence of extramedullary infiltrations (EMIs). EMIs can usually take the form of either diffuse organ infiltration (lungs, liver, spleen, gums, and meninges) or localized disease in the form of a mass (chloroma or granulocytic sarcoma), nodules/plaques in the skin (leukemia cutis), or other organs, identifiable clinically or by morphometabolic imaging.2
The clonal architecture of AML has mainly been studied in the bone marrow.3 However, the spatial diffusion in the body remains poorly understood. EMI is a frequent problem in the clinic, and its role in relapse after chemotherapy or after allogeneic stem cell transplantation is rarely explored in the literature. Indeed, as recently reviewed,4 cohort studies reported conflicting prognostic implications of EMI. In a large retrospective series of the ECOG-ACRIN Cancer Research Group Trials, from 1980 to 2008, no prognostic influence of EMI at diagnosis was found after adjustment with multivariate analysis.5 For all of these review studies, the morphologic assessment of EMI has to be made with caution as retrospective classification always carries the risk of bias.
Certain characteristics, including genomic (such as AML with t(8;21)(q22;q22.1) and AML with inv(16)(p13.1q22)) and/or CD56 adhesion protein expression, have long been known to have a greater propensity to disseminate outside the bone marrow, suggesting that features intrinsic to the leukemic cell may be responsible for extramedullary spread. Nonetheless, the observed correlations are not strong enough to have clinical or therapeutic implications at the individual level. Finally, as little was known about the mechanisms responsible for EMI, the relationship between leukemic and tissue stromal cells in this context was unknown, as was a possible biological link with leukemic resistance or persistence.
In this article, the authors took advantage of a case of relapsed AML, using leukemic skin lesions to decipher the intrinsic biological differences of blast cells according to their body distribution. Exploiting analysis of cells present in the bone marrow and the skin by single-cell RNA sequencing, the authors were able to uncover a subpopulation of blast cells with characteristics close to macrophages and whose transcriptome was significantly different in the skin than in the bone marrow. They found in this cluster particularly higher levels of C1q in the EMI lesion.
They then confirmed on tumor samples from other patients as well as on cohort data with publicly available genetic data that AML with EMI expressed higher levels of C1q and that this level increased with relapse. An association with Dnmt3a mutations, an unfavorable European LeukemiaNet (ELN)-2017 genetic profile, and a worse prognosis of C1q+ AML were also reported. Interestingly, the patient-derived xenograft (PDX) of C1q leukemic cells into immunodeficient mice confirmed the advantage of these cells to form extramedullary lesions (see figure). C1q blast cells did not show lower sensitivity to chemotherapy but had a greater capacity to migrate and adhere to the stroma. These blasts cells were stimulated by interaction with fibroblasts and the secretion of transforming growth factor-β (TGF-β), similar characteristics of macrophages associated with tumors. Coculture of fibroblasts and C1q leukemia cells increased resistance to chemotherapy and increased the secretion of extracellular matrix, which may also be involved in treatment resistance. Finally, Yang et al show that C1q is regulated by the transcription factors MAFB and TGF-β. However, it is not clear whether this deregulation is inherent to genetic alterations in tumor cells or related to external factors, such as the cytokine peritumoral environment, like interleukin-10 or TGF-β itself.
New findings in AML with extramedullary infiltration. Macrophage-like C1q blast cells were more frequent in extramedullary AML lesions, and these blasts cell were more prone to disseminate in a xenotransplantation experiment. Cytokine-mediated and direct interactions with tissue fibroblasts were implicated in chemotherapy resistance. Professional illustration by Patrick Lane, ScEYEnce Studios.
New findings in AML with extramedullary infiltration. Macrophage-like C1q blast cells were more frequent in extramedullary AML lesions, and these blasts cell were more prone to disseminate in a xenotransplantation experiment. Cytokine-mediated and direct interactions with tissue fibroblasts were implicated in chemotherapy resistance. Professional illustration by Patrick Lane, ScEYEnce Studios.
In recent years, several research studies have described new physiological functions (apart from the activation of the classic complement pathway) of the hexameric glycoprotein C1q, which is related to the tumor necrosis factor-α–like protein family.6 Recent studies have also shown that the globular C1q receptor (gC1qR) is an important regulator of metastasis formation via the activation of receptor tyrosine kinases in solid tumors.7 The work of Yang et al is of particular interest because it shows the role of fibroblast in leukemia. One may then wonder if macrophage-like C1q+ blast cells play a role close to the already famous macrophages associated with solid tumors, whose interaction with tumor-infiltrating fibroblasts participates in the resistance to various chemotherapies (ie, IL6R/STAT3 activation of tumor-infiltrating macrophages promotes resistance to 5-fluorouracil in a colorectal cancer model,8 or matrix metallopeptidase 9 (MMP-9) secretion by myeloid cells decreases capillary permeability and access to doxorubicin chemotherapy).9
This work raises many fascinating questions and opens a new field investigating the spatial dynamics of leukemia and exploring the link between dissemination and prognosis.
The question of the accessibility of tumor cells to chemotherapy in different organs is germane as it is already known that certain EMI sites are sanctuary sites, like the testis or the central nervous system. This could account for dissociated tumor responses between organs. Also, the involvement of mechanisms related to immunosurveillance deserves to be further investigated. Graft vs leukemia could be less effective outside the bone marrow, and relapse in the form of EMI (15% to 30%) has been observed after allogeneic stem cell transplantation.10 Finally, at present, leukemias remain the only cancers for which no tumor staging is performed at diagnosis or during relapses. We desperately lack reliable tools to measure the total tumor mass, which is probably extremely heterogeneous among patients because of a variable invasion of the blood and organs. It seems that it will be necessary to investigate this point as well in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests.