Abstract
Background: Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but fatal complication characterized by unusual site thrombosis followed by thrombocytopenia after the ChAdOx1 nCoV-19 vaccine. Heparin-induced thrombocytopenia antibodies or anti-platelet factor 4 (anti-PF4) antibodies have been identified as pathogenic antibodies in almost all patients. Data on anti-PF4 antibodies in Asian countries was still scarce.
Objectives: To determine the prevalence of anti-PF4 antibodies in the Thai population after administering the ChAdOx1 vaccine and the effect of the ChAdOx1 vaccine on anti-PF4 antibodies.
Methods: This study was a prospective cohort study. Healthy adults with aged ≥ 18 years were enrolled. Participants with thrombocytopenia (< 150 x 109/L), anticoagulant use, an immunocompromised state, or a recent major surgery were excluded. Blood samples were collected before and 4-6 weeks after the first dose of the ChAdOx1 vaccine. Participants with detectable anti-PF4 antibodies after the first dose of the ChAdOx1 vaccine were evaluated for clinical thrombosis, platelets, and D-dimer. They were also scheduled for the third blood sample to repeat anti-PF4 antibodies after 3 months of the second dose of the ChAdOx1 vaccine. Anti-PF4 antibodies were identified using Zymutest® HIA, IgG ELISA (Hyphen Biomed, Neuville-sur-Oise, France). We defined the negative cutoff optical density (OD) value was ≤ 0.3. The OD values of > 0.3 to 0.5, > 0.5 were assigned as weakly positive, and positive, respectively.
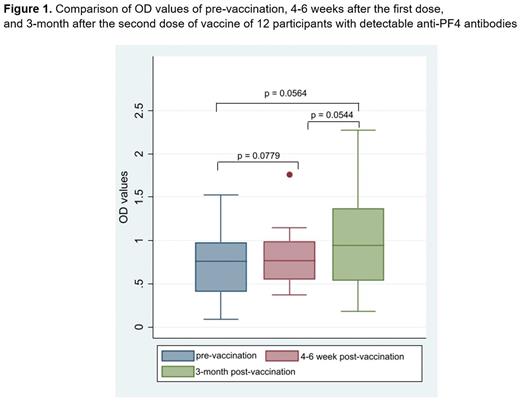
Results: A total of 396 participants were enrolled with a median age of 50 years (range 18-87). Of which, 65.91% were female and 8% were aspirin users. Twelve participants had detectable anti-PF4 antibodies after the first dose of the ChAdOx1 vaccine, given the prevalence of 3.03% (95% confidence interval [CI], 1.58-5.23), which was 3 participants (0.76%) in the weakly positive group. Ten of twelve participants (83.33%) had detectable anti-PF4 antibodies before the first dose of the ChAdOx1 vaccine. For participants with detectable anti-PF4 antibodies, only one participant (8.33%) had D-dimer greater than 500 ng/ml. There was no participant who had platelet counts less than 150x109/L. There was no significant difference of OD value in participants with detectable antibodies among pre-vaccination, 4-6 weeks after the first dose, and 3-month after the second dose of vaccine (Figure 1.). There were no participants with signs and symptoms of thrombosis after 1-month after both first and second dose of the ChAdOx1 vaccine.
Conclusion: The prevalence of pathogenic anti-PF4 antibodies and VITT after the ChAdOx1 vaccine was low in Thai people. There was minimal effect of the ChAdOx1 vaccine on the OD value of anti-PF4 antibodies, indicating that anti-PF4 antibodies should be tested only when VITT is suspected.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.