Abstract
Introduction: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an aggressive hematologic malignancy with a poor prognosis. It is characterized by clonal expansion of plasmacytoid dendritic tumor cells expressing specific markers, including the interleukin-3 (IL-3) receptor alpha (CD123). Tagraxofusp (TAG), approved by the FDA in December 2018, is a first-in-class CD123-targeted therapy comprising human IL-3 fused to a truncated diphtheria toxin payload. In August 2019, a global Expanded Access Program (EAP) was initiated in Europe to give patients (pts) access to and gather experience on the safety and efficacy of TAG in real-world practice. Herein we report preliminary results on 22 pts.
Aims: A European multicenter, noninterventional, retrospective study was conducted in pts with BPDCN treated with TAG in the EAP. Main objectives were rates of complete response (CR), and incidence and severity of capillary leak syndrome (CLS). Secondary objectives included rate of pts bridged to stem cell transplantation (SCT), progression-free survival (PFS), overall survival (OS), safety of TAG measured by the incidence and severity of adverse events (AEs), and number of TAG doses actually administered in each cycle.
Methods: The main inclusion criterion was a diagnosis of BPDCN, confirmed on histopathologic or cytologic samples using specific immunologic analyses with established marker panels (including CD123). The physician who signed the supply form of TAG in each participating center informed pts of their treatment. Training of physicians, nurses, and pharmacists was mandatory before initiation of treatment. The analysis included first-line (1L) and relapsed/refractory (R/R) pts enrolled in the EAP from August 2019 to December 2021. Pts received intravenous TAG at 12 mcg/kg once daily on days 1-5 (up to day 10 allowed) of a 21-day cycle. Hospitalization was required for the first cycle only; subsequent cycles could be administered in an outpatient setting.
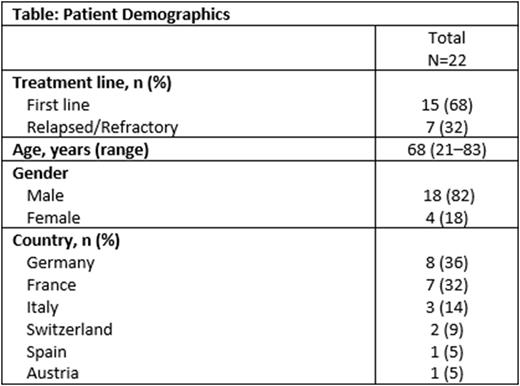
Results: These preliminary results are based on a database extraction date of June 30, 2022, comprising 22 pts. Pt demographics are shown in Table. Fifteen pts received TAG as 1L treatment and 7 pts were R/R. Median disease duration at time of TAG initiation was 2.7 months. Skin localization was documented in 18 pts (13 1L, 5 R/R). The median number of TAG cycles was 3: 4 (range 1-8) in 1L and 2 (range 1-5) in R/R pts, respectively. CR was reported in 10 of 15 1L pts (67%; 95% CI 38.4-88.2) and in 1 of 7 R/R pts (14%; 95% CI 0.4-57.9), and partial response (PR) was reported in 3 1L pts (20%) and in 3 R/R pts (43%); overall response rate (ORR) was 87% (95% CI 59.5-98.3) in 1L and 57% (95% CI 18.4-90.1) in R/R pts. Allogeneic SCT was undertaken in 9 of 15 1L pts (60%; 6 CR, 3 PR) and in 2 of 7 R/R pts (29%; 1 CR, 1 PR). With a median follow-up of 9.5 months (range 1.0-25.0), the median PFS was 7.8 months in 1L and 4.3 months in R/R pts. Median OS was not reached (NR) in 1L, and was 8.6 months in R/R pts. In SCT pts, median OS was NR both in 1L and R/R pts.
The majority of grade 3/4 AEs or serious AEs, regardless of their relationship to TAG therapy, occurred during cycle 1: 7 in 1L (47%) and 2 in R/R (29%) pts. Overall, CLS events were reported in 13 (59%) pts. The majority of CLS events (63%) were nonsevere (grade ≤2), with only 1 grade 4 in R/R pts and no grade 5 events. Median duration of CLS was 7 days and all CLS events resolved. Further analysis is ongoing.
Conclusion: This retrospective analysis of real-world clinical practice data is the first report in pts with BPDCN who were treated with TAG in Europe. The EAP included initial training provided to the multidisciplinary teams before treatment initiation. This is thought to have positively affected prevention, management, and resolution of CLS and other grade 3-4 AEs, which mainly occurred in cycle 1 in the inpatient setting; similar rates and severity were seen in older vs younger pts, and no death was related to CLS. Baseline characteristics did not appear to predispose pts to different treatment-related AEs. TAG in 1L pts resulted in an ORR of 87% including 67% CR, and 9 of 15 pts (60%) underwent allogeneic SCT; the median OS was NR in 1L pts with or without SCT.
Disclosures
Anant:Menarini Stemline: Current Employment. Paley:Stemline Therapeutics: Current Employment. Riggi:Menarini/Stemline Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Herling:Stemline Therapeutics: Consultancy; AbbVie: Consultancy; BeiGene: Consultancy; Jazz: Consultancy; Takeda: Consultancy; Mundipharma EDO: Research Funding; Janpix: Research Funding; Novartis: Research Funding; Roche: Research Funding. Angelucci:Celgene: Honoraria, Other: Data monitoring committee; Bluebird Bio: Consultancy; Glaxo: Consultancy; Gilead: Consultancy; Roche: Consultancy; Vertex: Honoraria, Other: Data monitoring committee; Sanofi: Speakers Bureau; Vifopr: Honoraria, Other: Data monitoring committee; Novartis: Honoraria; Menarini/Stemline: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.