Abstract
INTRODUCTIONDiffuse Large B-cell Lymphoma (DLBCL) is most frequently diagnosed in the elderly, with a median age of presentation of 66 years. Anthracycline - based chemoimmunotherapy is associated with cardiotoxicity and may not always be safe in frail patients or in patients ≥ 85 years. Little is known about treatment patterns and outcomes of the oldest DLBCL patients.
METHODS: We used the Surveillance, Epidemiology, and End Results Program (SEER) linked with Medicare claims to identify 6,214 DLBCL patients diagnosed between 2007 and 2017. Data collected included demographic variables, comorbidity burden calculated using the Elixhauser comorbidity index with conditions present 30 to 365 days prior to DLBCL diagnosis. Initial therapy was defined as prescribed -30 to 100 days post-diagnosis to account for potential database date issues. Patients who did not receive outpatient treatment but had current procedural terminology codes reflecting inpatient chemotherapy were accounted for in a separate group. We created 5 groups: anthracycline, non - anthracycline, inpatient-only treatment, palliative therapy (steroids, rituximab), and no treatment.
RESULTS: Of the 6,214 patients, 1,366 (22%) were ≥ 85 years old. In these older patients 22.5% (n=300) received treatment with an anthracycline-based regimen, 14.1% received treatment without an anthracycline (n=192), 7.2% received inpatient only chemotherapy (n=99), 13.2% received palliative treatment and the remaining 43.6% received no treatment. This contrasts with younger patients (< 85 y), in which 53.3% received anthracyclines.
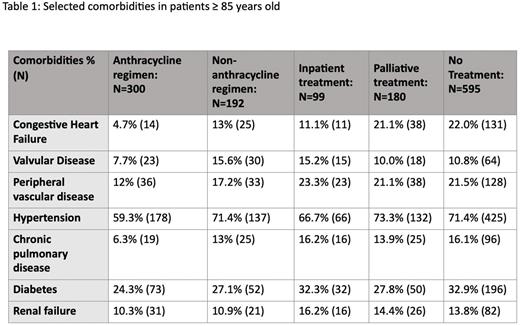
Table 1 lists baseline comorbidities of patients ≥ 85 years old. Congestive heart failure was less frequent among anthracycline-treated patients (4.7%) vs. non-anthracycline (13%), palliative therapy (21.1%), or no treatment group (22.0%).
Median survival varied greatly across treatment groups. Untreated patients in the oldest-old group had a median survival of 2 months (95% CI 2-3 months) vs. 7.5 months (95% CI 5-10 months) for patients receiving palliative therapy, 13 months (95% CI 11-19 months) for non-anthracycline - treated patients and 45 months (95% CI 34-54 months) for anthracycline - treated patients. Patients who only received inpatient chemotherapy had a median survival of 2 months (95% CI 2-4 months).
In a Cox regression analysis adjusting for sex, stage, and Elixhauser comorbidity index, anthracycline use was associated with an hazard ratio (HR) of 0.22 compared to untreated patients (HR, 95% CI 0.18-0.26). The HR with non-anthracycline chemotherapy use was 0.41 (95% CI 0.34-0.49) compared to untreated patients. Patients treated with palliative treatment and inpatient only treatments had an HR for survival of 0.59 (95% 0.49-0.70) and 0.72 (95% CI 0.57-0.92) compared to untreated patients, respectively.
CONCLUSIONS: Our findings show DLBCL patients older than 85 years of age can achieve durable remissions and can experience disease cure if they are treated with effective agents. In this group of oldest old patients, anthracycline - based treatments were associated with significantly longer survival than other therapies, even after adjusting for comorbidities. Patients not receiving therapy and those receiving palliative approaches have very short survival times. These analyses highlight the need for novel effective therapies that can be tolerated by the large proportion of DLBCL patients ≥ 85 years who cannot be prescribed an anthracycline containing regimen.
Disclosures
Caimi:Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.