Abstract
Introduction: AB-101 is a non-engineered, allogeneic, off-the-shelf, cryopreserved cord blood-derived natural killer (NK)-cell therapy in development as a cancer therapeutic. A highly scaled manufacturing process enables production of 1000s of doses of AB-101 from a single donor cord blood unit (CBU). AB-101 is derived from CBUs selected for KIR-B haplotype and natural high-affinity variant of CD16 (158V/V), which are associated with improved anti-tumor activity and ADCC enhancement, resulting in a highly active NK cell product without the requirement for additional engineering. We are developing AB-101 for combination with monoclonal antibodies (mAbs) to enhance antibody dependent cellular cytotoxicity (ADCC) in patients, initially in an ongoing Phase I clinical trial for NHL. To further develop AB-101 as an ADCC enhancer, we assessed anti-tumor activity in combination with CD38 mAb, against multiple myeloma (MM) cell lines. CD38 is a transmembrane glycoprotein that is uniformly and highly upregulated on MM cells. Anti-CD38 mAbs exert anti-tumor activity against MM through ADCC and other mechanisms. NK cells play a key role in ADCC responses through CD16 receptor signaling. However, the majority of NK cells express CD38 and are depleted by therapeutic CD38 mAbs which could limit their therapeutic efficacy. Combining NK cell therapies with anti-CD38 mAbs, thus necessitates CD38 knockout to prevent fratricide. AB-101 has a naturally low percent and intensity of CD38 expression. Here we present data demonstrating that AB-101 are resistant to anti-CD38 mAb-induced fratricide and can elicit significant ADCC against MM cell lines both in vitro and in vivo. Since anti-CD38 mAbs are combined with glucocorticoids in the clinic, we assessed the impact of dexamethasone and methylprednisolone on ADCC by AB-101. Here we report robust ADCC following treatment of AB-101 with glucocorticoids.
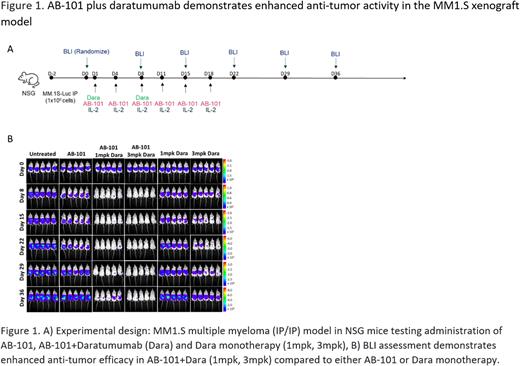
Methods: AB-101 was screened for CD38 expression and incubated with anti-CD38 mAb - daratumumab or hIgG1 control (300 - 0.41 µg/ml) for 4 hours to assess fratricide. In vitro ADCC assays were performed at various effector to target ratios against control (Daudi and K562) and MM cell lines (NCI-H929, RPMI 8226, MM.1S and MM.1R) using 2 µg/ml of daratumumab or hIgG1. To study the effect of glucocorticoids, AB-101 was cultured in various concentrations of dexamethasone and methylprednisolone for 48 hours in the presence of 50 or 500 IU/ml of IL-2, prior to ADCC assay. In vivo efficacy of AB-101 in combination with daratumumab was assessed in the intraperitoneal (IP) MM.1S-Luc xenograft model in NSG mice (Figure 1A). Daratumumab and AB-101 were administered by IP injection. Tumor burden was assessed by bioluminescent imaging (BLI).
Results: AB-101 was over 98% CD56+/CD3- with 87% CD56+/CD16+ population. Only 22% of GMP drug product were CD38+ with low intensity of expression compared to peripheral blood NK cells (~88%). In the presence of daratumumab AB-101 viability was unaltered compared to hIgG1 control demonstrating resistance to fratricide. Daratumumab enhanced ADCC potential of AB-101 against MM cell lines and Daudi in in vitro. ADCC against MM lines and Daudi did not decrease on exposure to glucocorticoids compared to no treatment group. In vivo anti-tumor efficacy, as evidenced by reduction in BLI signal, was observed in the MM.1S model in AB-101 + daratumumab groups compared to daratumumab and AB-101 alone (Figure 1B). AB-101 + daratumumab (1 and 3mpk) led to 98 and 99% tumor growth inhibition (TGI), respectively, relative to no treatment. Daratumumab monotherapy at 1 and 3mpk showed 21% and 87% TGI respectively, and AB-101 showed 16% TGI relative to no treatment.
Conclusions: AB-101 has high and consistent expression of CD16 and low CD38 expression and is resistant to daratumumab induced fratricide and hence can be combined with daratumumab without additional engineering such as CD38 knockout. Daratumumab is combined in the clinic with glucocorticoids, immunomodulatory drugs (IMiDs) and proteosome inhibitors (PI). While IMiDs and PIs are reported to enhance NK cell function, glucocorticoids have been shown to diminish NK cell cytotoxicity. Data presented here suggests that ADCC potential of AB-101 is not negatively impacted by glucocorticoids. Taken together, the in vitro and in vivo data supports further clinical development of AB-101 in combination with daratumumab to treat MM patients.
Disclosures
Chu:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Teng:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Guerrettaz:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Da Silva:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Rotondo:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. La Torre:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Conerty:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Flynn:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Seto:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Raymon:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company. Somanchi:Artiva Biotherapeutics: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.